 Standardization is the activity of establishing rules and characteristics for the purpose of their voluntary reuse, aimed at achieving orderliness in the areas of production and circulation of products and increasing competitiveness.
Standardization is the activity of establishing rules and characteristics for the purpose of their voluntary reuse, aimed at achieving orderliness in the areas of production and circulation of products and increasing competitiveness.
 Standardization in the field of drug circulation is aimed at ensuring the quality assurance of a standardized object, which includes products (raw materials and materials, finished drugs, etc.), services, processes.
Standardization in the field of drug circulation is aimed at ensuring the quality assurance of a standardized object, which includes products (raw materials and materials, finished drugs, etc.), services, processes.
 Standardization in relation to the field of drug circulation is the process of establishing and applying standards that contain a set of norms, rules, requirements for the object of standardization, including all medicines, including homeopathic, parapharmaceutical products, services and processes in the field of drug circulation, guaranteeing their quality.
Standardization in relation to the field of drug circulation is the process of establishing and applying standards that contain a set of norms, rules, requirements for the object of standardization, including all medicines, including homeopathic, parapharmaceutical products, services and processes in the field of drug circulation, guaranteeing their quality.
 The purpose of standardization in the field of drug circulation is to protect consumers and the state on the quality of pharmaceutical products, the processes of their production, promotion, storage, destruction, etc. Thus, standardization is aimed at ensuring pharmacological, environmental, technological safety, rational use of resources.
The purpose of standardization in the field of drug circulation is to protect consumers and the state on the quality of pharmaceutical products, the processes of their production, promotion, storage, destruction, etc. Thus, standardization is aimed at ensuring pharmacological, environmental, technological safety, rational use of resources.
 Principles implemented during standardization: 1. Voluntary use of standards and provision of conditions for their uniform application. 2. The use of an international standard as the basis for the development of a national standard, except in cases where compliance with the requirements international standards impossible due to the inconsistency of their requirements with the climatic and geographical features of the Russian Federation or technical (technological) features. In particular, the draft general pharmacopeial articles for the XII State Pharmacopoeia take into account the modern requirements of foreign pharmacopoeias.
Principles implemented during standardization: 1. Voluntary use of standards and provision of conditions for their uniform application. 2. The use of an international standard as the basis for the development of a national standard, except in cases where compliance with the requirements international standards impossible due to the inconsistency of their requirements with the climatic and geographical features of the Russian Federation or technical (technological) features. In particular, the draft general pharmacopeial articles for the XII State Pharmacopoeia take into account the modern requirements of foreign pharmacopoeias.
 Principles implemented in the course of standardization: 3. Inadmissibility of creating obstacles to the production and circulation of products, the performance of work and the provision of services to a greater extent than is minimally necessary to achieve the goals of standardization. 4. Balance of interests of the parties that develop, provide and consume products (services). This principle means that the participants in the standardization work, based on the capabilities of the product manufacturer and service provider, on the one hand, and the requirements of the consumer, on the other, must find a consensus, which is understood as a general agreement. In other words: the lack of objections on significant issues from the majority of stakeholders, the desire to take into account the opinions of all parties and bring together dissenting points of view. 5. Consistency of standardization, which involves considering the object of standardization as part of a more complex system.
Principles implemented in the course of standardization: 3. Inadmissibility of creating obstacles to the production and circulation of products, the performance of work and the provision of services to a greater extent than is minimally necessary to achieve the goals of standardization. 4. Balance of interests of the parties that develop, provide and consume products (services). This principle means that the participants in the standardization work, based on the capabilities of the product manufacturer and service provider, on the one hand, and the requirements of the consumer, on the other, must find a consensus, which is understood as a general agreement. In other words: the lack of objections on significant issues from the majority of stakeholders, the desire to take into account the opinions of all parties and bring together dissenting points of view. 5. Consistency of standardization, which involves considering the object of standardization as part of a more complex system.
 Principles implemented during standardization: 6. Dynamism and advanced development of the standard - a document that establishes the characteristics of products and processes for their production, operation, storage, transportation, sale and disposal, as well as the rules for their implementation. Dynamism is ensured by periodic revision of regulatory documents, amendments to them or cancellation. Advanced development is facilitated by the introduction into the standard of promising requirements for products, processes, methods of their control, quality indicators, etc. 7. The effectiveness of standardization, expressed in economic or social effect. 8. Harmonization of standards with the requirements of technical regulations. 9. Clarity and unambiguity of the wording of the provisions of the standard. 10. Complexity of standardization of interconnected objects. 11. Objectivity of verification of requirements.
Principles implemented during standardization: 6. Dynamism and advanced development of the standard - a document that establishes the characteristics of products and processes for their production, operation, storage, transportation, sale and disposal, as well as the rules for their implementation. Dynamism is ensured by periodic revision of regulatory documents, amendments to them or cancellation. Advanced development is facilitated by the introduction into the standard of promising requirements for products, processes, methods of their control, quality indicators, etc. 7. The effectiveness of standardization, expressed in economic or social effect. 8. Harmonization of standards with the requirements of technical regulations. 9. Clarity and unambiguity of the wording of the provisions of the standard. 10. Complexity of standardization of interconnected objects. 11. Objectivity of verification of requirements.
 Stages of the standardization procedure 1. Selection of objects of standardization. 2. Description of the object, taking into account the basic requirements, i.e. its modeling. 3. Optimization of the model in terms of unification of the object using the methods of systematization, typification, simplification (identification of inappropriate objects), etc. 4. Standardization of the model - development of a regulatory document (RD) based on a unified model.
Stages of the standardization procedure 1. Selection of objects of standardization. 2. Description of the object, taking into account the basic requirements, i.e. its modeling. 3. Optimization of the model in terms of unification of the object using the methods of systematization, typification, simplification (identification of inappropriate objects), etc. 4. Standardization of the model - development of a regulatory document (RD) based on a unified model.
 Thus, the final document of standardization is a normative document that establishes the rules general principles or characteristics of various activities or their results. The term "Regulatory document" is generic and covers such concepts as standards and other normative documents on standardization: rules, recommendations, codes of practice, all-Russian classifiers.
Thus, the final document of standardization is a normative document that establishes the rules general principles or characteristics of various activities or their results. The term "Regulatory document" is generic and covers such concepts as standards and other normative documents on standardization: rules, recommendations, codes of practice, all-Russian classifiers.
 A standard is a standard or sample taken as a reference for comparing other similar objects with it. The standard as ND establishes a set of norms and requirements for the object of standardization. The application of standards contributes to the improvement of product quality. Standard - a document in which, for the purpose of voluntary reuse, product characteristics, implementation rules and characteristics of processes, production, operation, storage, transportation, sale and disposal, performance of work or provision of services are established. The standard may contain requirements for terminology, symbols, packaging, marking or labels and the rules for their application.
A standard is a standard or sample taken as a reference for comparing other similar objects with it. The standard as ND establishes a set of norms and requirements for the object of standardization. The application of standards contributes to the improvement of product quality. Standard - a document in which, for the purpose of voluntary reuse, product characteristics, implementation rules and characteristics of processes, production, operation, storage, transportation, sale and disposal, performance of work or provision of services are established. The standard may contain requirements for terminology, symbols, packaging, marking or labels and the rules for their application.
 Classification of standards depending on the scope 1. international, adopted by an international organization. 2. national, approved by the national body of the Russian Federation for standardization. 3. state (industry), put into effect by the state executive authority. For example, state educational standards are the area of competence of the Ministry of Education of the Russian Federation. 4. enterprise standard.
Classification of standards depending on the scope 1. international, adopted by an international organization. 2. national, approved by the national body of the Russian Federation for standardization. 3. state (industry), put into effect by the state executive authority. For example, state educational standards are the area of competence of the Ministry of Education of the Russian Federation. 4. enterprise standard.
 Medicinal product quality standard is a regulatory document containing a list of standardized indicators and methods for drug quality control, approved by the sectoral ministry.
Medicinal product quality standard is a regulatory document containing a list of standardized indicators and methods for drug quality control, approved by the sectoral ministry.
 Quality standards for medicines The provisions of the standards are mandatory for drug development organizations and manufacturers departmental affiliation, legal status and forms of ownership, as well as for organizations and institutions that carry out examination of drug quality standards.
Quality standards for medicines The provisions of the standards are mandatory for drug development organizations and manufacturers departmental affiliation, legal status and forms of ownership, as well as for organizations and institutions that carry out examination of drug quality standards.
 The standard does not apply to the development and production of pharmaceutical products: - from blood and its substitutes used for transfusiology; - from raw materials of animal origin, used only for the preparation of products subject to further industrial processing for the preparation of drugs; - foreign production; - manufactured in pharmacies.
The standard does not apply to the development and production of pharmaceutical products: - from blood and its substitutes used for transfusiology; - from raw materials of animal origin, used only for the preparation of products subject to further industrial processing for the preparation of drugs; - foreign production; - manufactured in pharmacies.
 Quality standards that establish requirements for drugs are divided into the following categories: - State quality standards for drugs (GSKLS); - Pharmacopoeia article of the enterprise (FSP) on the drugs of a particular enterprise.
Quality standards that establish requirements for drugs are divided into the following categories: - State quality standards for drugs (GSKLS); - Pharmacopoeia article of the enterprise (FSP) on the drugs of a particular enterprise.
 State quality standards Ø general pharmacopoeial article (GPM) - includes a list of normalized indicators or test methods for a specific dosage form, a description of physical, physico-chemical, biochemical, biological, microbiological methods of drug analysis, requirements for the reagents used, titrated solutions, indicators. Ø pharmacopoeial monograph (FS). Developed for monocomponent drugs by international non-proprietary name (INN) and contains mandatory list indicators and methods of drug quality control (taking into account its dosage form) that meet the requirements of leading foreign pharmacopoeias. Ø OFS and FS constitute the State Pharmacopoeia (SP).
State quality standards Ø general pharmacopoeial article (GPM) - includes a list of normalized indicators or test methods for a specific dosage form, a description of physical, physico-chemical, biochemical, biological, microbiological methods of drug analysis, requirements for the reagents used, titrated solutions, indicators. Ø pharmacopoeial monograph (FS). Developed for monocomponent drugs by international non-proprietary name (INN) and contains mandatory list indicators and methods of drug quality control (taking into account its dosage form) that meet the requirements of leading foreign pharmacopoeias. Ø OFS and FS constitute the State Pharmacopoeia (SP).
 GF - compilation state standards quality of drugs, which is of a legislative nature. The Global Fund is based on the principles of national health care and reflects modern achievements in the field of pharmacy, medicine, chemistry and other related sciences. The Global Fund is an integral part of a comprehensive system for ensuring the quality of pharmaceutical products, its requirements are mandatory for all enterprises and institutions involved in the field of drug circulation, regardless of ownership and departmental subordination.
GF - compilation state standards quality of drugs, which is of a legislative nature. The Global Fund is based on the principles of national health care and reflects modern achievements in the field of pharmacy, medicine, chemistry and other related sciences. The Global Fund is an integral part of a comprehensive system for ensuring the quality of pharmaceutical products, its requirements are mandatory for all enterprises and institutions involved in the field of drug circulation, regardless of ownership and departmental subordination.
 One of the fundamental standards of this level is the International Pharmacopoeia (IP), which establishes acceptable standards for the potency, purity and quality of pharmaceutical products entering the market. international market. MF is an alternative to some of the widely applicable national and regional pharmacopoeias. Its main goal is to adapt to the needs of developing countries by offering reliable quality standards based on classical methods, thus providing state guarantees for medicines. The main guidelines for the development of domestic state quality standards for medicinal products are the European, British Pharmacopoeia and US Pharmacopoeia.
One of the fundamental standards of this level is the International Pharmacopoeia (IP), which establishes acceptable standards for the potency, purity and quality of pharmaceutical products entering the market. international market. MF is an alternative to some of the widely applicable national and regional pharmacopoeias. Its main goal is to adapt to the needs of developing countries by offering reliable quality standards based on classical methods, thus providing state guarantees for medicines. The main guidelines for the development of domestic state quality standards for medicinal products are the European, British Pharmacopoeia and US Pharmacopoeia.
 The pharmacopoeial article of the enterprise (FSP) is a quality standard for medicine under a trade name, containing a list of indicators and methods for quality control of a medicinal product produced by a particular enterprise, taking into account the specific technology of this enterprise and duly registered.
The pharmacopoeial article of the enterprise (FSP) is a quality standard for medicine under a trade name, containing a list of indicators and methods for quality control of a medicinal product produced by a particular enterprise, taking into account the specific technology of this enterprise and duly registered.
 The FSP, created by the development organization or manufacturing enterprise in relation to its production, is the object of their copyright. The FSP contains a list of quality indicators of a substance or medicinal product manufactured by a given enterprise, with a detailed description of the methods for its assessment. Thus, this document reflects the specific technology of a particular enterprise in the manufacture specific products, passed the examination and state registration. The norms specified in the FSP can be much stricter, but not lower than those requirements provided for in the OFS. Enterprise standards may differ from the FS by using other alternative more economical, faster, automated or more accurate methods of analysis and testing; introduction of additional characteristics; inclusion detailed description sampling, which ensures a higher level of standardization of serial products; using tighter tolerances and limits to create a margin of safety, reduce the risk of the result being out of range due to batch heterogeneity or method error. The validity period of the FSP is established upon its approval, taking into account the level of the technological process of a particular drug production, but not more than 5 years.
The FSP, created by the development organization or manufacturing enterprise in relation to its production, is the object of their copyright. The FSP contains a list of quality indicators of a substance or medicinal product manufactured by a given enterprise, with a detailed description of the methods for its assessment. Thus, this document reflects the specific technology of a particular enterprise in the manufacture specific products, passed the examination and state registration. The norms specified in the FSP can be much stricter, but not lower than those requirements provided for in the OFS. Enterprise standards may differ from the FS by using other alternative more economical, faster, automated or more accurate methods of analysis and testing; introduction of additional characteristics; inclusion detailed description sampling, which ensures a higher level of standardization of serial products; using tighter tolerances and limits to create a margin of safety, reduce the risk of the result being out of range due to batch heterogeneity or method error. The validity period of the FSP is established upon its approval, taking into account the level of the technological process of a particular drug production, but not more than 5 years.
 Examination of all created and revised ND is carried out by the Pharmacopoeial Committee. If necessary, in agreement with the manufacturer, specialized organizations may be involved in conducting experimental checks of drug quality standards. During the examination, they check: - the scientific and technical level of the draft standard and its compliance modern requirements GF XI and GF XII editions; - Validity of the list of quality indicators; - optimality of values of quality standards and shelf life of medicines; - accuracy and unambiguity of used terms, definitions, chemical nomenclature and units of physical quantities.
Examination of all created and revised ND is carried out by the Pharmacopoeial Committee. If necessary, in agreement with the manufacturer, specialized organizations may be involved in conducting experimental checks of drug quality standards. During the examination, they check: - the scientific and technical level of the draft standard and its compliance modern requirements GF XI and GF XII editions; - Validity of the list of quality indicators; - optimality of values of quality standards and shelf life of medicines; - accuracy and unambiguity of used terms, definitions, chemical nomenclature and units of physical quantities.
 An integral part of the set of documentation submitted to the Pharmacopoeia Committee is a brief scheme for the synthesis of drugs with an indication of the solvents used in the synthesis process, as well as at the last stage of preparation and purification. OFS, FS and FSP, after approval, are registered with an organization authorized for this by the Ministry of Health of Russia, with the assignment of a designation. The OFS and FS designations consist of the abbreviated name of the drug quality standard category, the index of the Ministry of Health of Russia, the registration number assigned to the document, and the last two digits of the year of approval or revision, separated by a dash. The FSP designation, in addition to the listed parameters, includes an indication of a four-digit enterprise code before the registration number of the document. The enterprise code is assigned when applying for approval of the first FSP. Amendments to the quality standards of drugs of all categories are made in cases where an increase in the scientific and / or technological level allows improving the quality of drugs or clarifying quality indicators.
An integral part of the set of documentation submitted to the Pharmacopoeia Committee is a brief scheme for the synthesis of drugs with an indication of the solvents used in the synthesis process, as well as at the last stage of preparation and purification. OFS, FS and FSP, after approval, are registered with an organization authorized for this by the Ministry of Health of Russia, with the assignment of a designation. The OFS and FS designations consist of the abbreviated name of the drug quality standard category, the index of the Ministry of Health of Russia, the registration number assigned to the document, and the last two digits of the year of approval or revision, separated by a dash. The FSP designation, in addition to the listed parameters, includes an indication of a four-digit enterprise code before the registration number of the document. The enterprise code is assigned when applying for approval of the first FSP. Amendments to the quality standards of drugs of all categories are made in cases where an increase in the scientific and / or technological level allows improving the quality of drugs or clarifying quality indicators.
 For example, FS 42 -000 056 - 01 stands for: FS - standard category - pharmacopoeial article; 42 - index of the Ministry of Health of Russia to designate standardization documents; 000 056 - registration number of the document; 01 - the last two digits of the year of approval (2001).
For example, FS 42 -000 056 - 01 stands for: FS - standard category - pharmacopoeial article; 42 - index of the Ministry of Health of Russia to designate standardization documents; 000 056 - registration number of the document; 01 - the last two digits of the year of approval (2001).
 Standardization Methods A standardization method is a technique or a set of techniques by which the goals of standardization are achieved. A universal method in the field of standardization of products, processes and services is the ordering of standardization objects through the management of diversity. Ordering as a universal method consists of separate methods: systematization, selection, simplification, typing and optimization.
Standardization Methods A standardization method is a technique or a set of techniques by which the goals of standardization are achieved. A universal method in the field of standardization of products, processes and services is the ordering of standardization objects through the management of diversity. Ordering as a universal method consists of separate methods: systematization, selection, simplification, typing and optimization.
 The systematization of standardization objects consists in a scientifically based, consistent classification and ranking of a set of specific standardization objects. An example of the result of work on the systematization of products is All-Russian classifier products (OKP), which systematizes the entire marketable products by industry in the form of various classification groups and specific product names.
The systematization of standardization objects consists in a scientifically based, consistent classification and ranking of a set of specific standardization objects. An example of the result of work on the systematization of products is All-Russian classifier products (OKP), which systematizes the entire marketable products by industry in the form of various classification groups and specific product names.
 Selection and siplification of standardization objects is an activity that consists in the selection and identification of specific objects that are recognized as appropriate for further production and use (for example, expert review MD, which should be included in the list of vital and essential drugs). Selection and siplification processes are carried out in parallel. They are preceded by classification, ranking of objects, a special analysis of the prospects and comparison of objects with future needs.
Selection and siplification of standardization objects is an activity that consists in the selection and identification of specific objects that are recognized as appropriate for further production and use (for example, expert review MD, which should be included in the list of vital and essential drugs). Selection and siplification processes are carried out in parallel. They are preceded by classification, ranking of objects, a special analysis of the prospects and comparison of objects with future needs.
 Typification of standardization objects is an activity to create standard (exemplary) objects, technological rules, documentation forms. Optimization of standardization objects consists in finding the optimal main parameters (destination parameters), as well as the values of all other quality and economy indicators. Optimization of standardization objects is carried out by applying special economic and mathematical methods and optimization models. The goal of optimization is to achieve the optimal degree of order and the maximum possible efficiency according to the selected criterion.
Typification of standardization objects is an activity to create standard (exemplary) objects, technological rules, documentation forms. Optimization of standardization objects consists in finding the optimal main parameters (destination parameters), as well as the values of all other quality and economy indicators. Optimization of standardization objects is carried out by applying special economic and mathematical methods and optimization models. The goal of optimization is to achieve the optimal degree of order and the maximum possible efficiency according to the selected criterion.
 Standard samples According to the purpose, the following types of comparison samples are distinguished: - samples for assessing the accuracy of the analysis - they are used to judge the correctness, and sometimes the precision of the analysis; - calibration samples are used in the construction of a calibration characteristic; RMs, depending on the purpose of application, are divided into groups: 1. State standard samples (SRM); 2. Working standard samples (RSS); 3. Standard samples of substances-witnesses (SWS).
Standard samples According to the purpose, the following types of comparison samples are distinguished: - samples for assessing the accuracy of the analysis - they are used to judge the correctness, and sometimes the precision of the analysis; - calibration samples are used in the construction of a calibration characteristic; RMs, depending on the purpose of application, are divided into groups: 1. State standard samples (SRM); 2. Working standard samples (RSS); 3. Standard samples of substances-witnesses (SWS).
 GSO - specially prepared compounds high degree purity, the standard quality indicators of which are reflected in the FS and meet the WHO requirements for this RM. They are used to identify drugs by IR spectroscopy, chromatographic methods, determine specific impurities and quantification medicinal substances (substances) by HPLC, photometry and UV spectrophotometry. Most often used in assessing the quality of medicinal substances-substances.
GSO - specially prepared compounds high degree purity, the standard quality indicators of which are reflected in the FS and meet the WHO requirements for this RM. They are used to identify drugs by IR spectroscopy, chromatographic methods, determine specific impurities and quantification medicinal substances (substances) by HPLC, photometry and UV spectrophotometry. Most often used in assessing the quality of medicinal substances-substances.
 RSO - serial medicinal substances that meet pharmacopoeial requirements. They are intended for the determination of medicinal substances by physicochemical methods in dosage forms, while CO in the calculations of the quantitative content is taken as 100%, as well as for conducting individual tests when assessing the quality of substances. Used to analyze dosage forms.
RSO - serial medicinal substances that meet pharmacopoeial requirements. They are intended for the determination of medicinal substances by physicochemical methods in dosage forms, while CO in the calculations of the quantitative content is taken as 100%, as well as for conducting individual tests when assessing the quality of substances. Used to analyze dosage forms.
 Standard samples of substances-witnesses (SWS) - are used to determine impurities or establish the component composition of drugs. GSO RSO or other specially manufactured and certified substances can be used as SSMS.
Standard samples of substances-witnesses (SWS) - are used to determine impurities or establish the component composition of drugs. GSO RSO or other specially manufactured and certified substances can be used as SSMS.
 Standard samples are used in the following types of analytical techniques: - IR spectroscopy for drug identification; - UV spectrophotometry for quantitative determination; - quantitative determination based on the measurement of color intensity; - chromatographic separation methods for identification and quantification; - quantitative methods, based on separation methods, depending on the distribution of the analyte between the solvent phases; - polarographic and polarimetric methods.
Standard samples are used in the following types of analytical techniques: - IR spectroscopy for drug identification; - UV spectrophotometry for quantitative determination; - quantitative determination based on the measurement of color intensity; - chromatographic separation methods for identification and quantification; - quantitative methods, based on separation methods, depending on the distribution of the analyte between the solvent phases; - polarographic and polarimetric methods.
 The introduction of RM into the practice of pharmaceutical analysis allows achieving a number of positive solutions: 1. Apply latest methods research. 2. Ensure the uniformity of measurements. It is known that the results of the analysis of the same drug, carried out on different instruments, differing in sensitivity and resolution, can give significant discrepancies. It is possible to exclude the influence of an instrumental error using RM, since the content of substances in the standards is known and the determination is reduced to comparing the analyzed preparation with the standard under the same conditions. 3. Simplify and speed up measurements, which follows from the previous one.
The introduction of RM into the practice of pharmaceutical analysis allows achieving a number of positive solutions: 1. Apply latest methods research. 2. Ensure the uniformity of measurements. It is known that the results of the analysis of the same drug, carried out on different instruments, differing in sensitivity and resolution, can give significant discrepancies. It is possible to exclude the influence of an instrumental error using RM, since the content of substances in the standards is known and the determination is reduced to comparing the analyzed preparation with the standard under the same conditions. 3. Simplify and speed up measurements, which follows from the previous one.
 The introduction of RM into the practice of pharmaceutical analysis makes it possible to achieve a number of positive solutions: 4. To increase the accuracy and reliability of analysis methods. 5. Develop automatic quality control methods. 6. Achieve the correctness of the results of measurements of quantities characterizing the composition and properties of substances and the possibility of reliable calibration of analytical instruments.
The introduction of RM into the practice of pharmaceutical analysis makes it possible to achieve a number of positive solutions: 4. To increase the accuracy and reliability of analysis methods. 5. Develop automatic quality control methods. 6. Achieve the correctness of the results of measurements of quantities characterizing the composition and properties of substances and the possibility of reliable calibration of analytical instruments.
 A reference sample is a substance used to ensure the quality of measurements (calibration, certification of methods, evaluation of the statistical characteristics of the measuring process, etc.) Comparison samples can be conditionally divided into comparison samples of simple and complex composition.
A reference sample is a substance used to ensure the quality of measurements (calibration, certification of methods, evaluation of the statistical characteristics of the measuring process, etc.) Comparison samples can be conditionally divided into comparison samples of simple and complex composition.
 Comparison samples of simple composition are pure substances and their solutions. Comparison samples of complex composition are called multicomponent samples that are close to the composition and properties of routine samples. They are prepared on the basis of substances of the same nature with routine samples.
Comparison samples of simple composition are pure substances and their solutions. Comparison samples of complex composition are called multicomponent samples that are close to the composition and properties of routine samples. They are prepared on the basis of substances of the same nature with routine samples.

 The stability of reference samples lies in the fact that the change in the determined parameters (concentration of analytes) from the moment of certification to the moment of use should be small compared to the error of the reference sample. Special handling is often needed to ensure stability. For example, substances that prevent their oxidation and have bactericidal properties are added to solutions of organic substances (glucose solution is stabilized with benzoic acid); liquids are hermetically sealed to avoid evaporation (soldered into ampoules); unstable samples are stored and sent at a low temperature, lyophilized, etc.
The stability of reference samples lies in the fact that the change in the determined parameters (concentration of analytes) from the moment of certification to the moment of use should be small compared to the error of the reference sample. Special handling is often needed to ensure stability. For example, substances that prevent their oxidation and have bactericidal properties are added to solutions of organic substances (glucose solution is stabilized with benzoic acid); liquids are hermetically sealed to avoid evaporation (soldered into ampoules); unstable samples are stored and sent at a low temperature, lyophilized, etc.
 The stability of reference samples is usually expressed in the form of its shelf life - the time during which the error associated with instability does not exceed an acceptable value. Homogeneity (homogeneity) of reference samples is a property expressed in the constancy of the concentration of analytes. Adequacy is usually required for comparison samples of complex composition and lies in the fact that they are analytically close to routine samples.
The stability of reference samples is usually expressed in the form of its shelf life - the time during which the error associated with instability does not exceed an acceptable value. Homogeneity (homogeneity) of reference samples is a property expressed in the constancy of the concentration of analytes. Adequacy is usually required for comparison samples of complex composition and lies in the fact that they are analytically close to routine samples.
 Control and archive samples The presence of control and archive samples is necessary for two purposes: 1. to ensure the availability of a sample for analytical studies; 2. To ensure that the sample is complete finished products.
Control and archive samples The presence of control and archive samples is necessary for two purposes: 1. to ensure the availability of a sample for analytical studies; 2. To ensure that the sample is complete finished products.
 A control sample is a sample taken from a batch of raw materials, packaging material or finished products, which is stored for analysis during the expiration date of the batch, if necessary. Samples taken at critical intermediate stages (for example, after which analytical studies and release approvals are expected) and samples of intermediates that are shipped outside the manufacturer's control area should be retained, if the stability of the samples allows this. Designed for analysis and should be readily available to a laboratory that has validated methods for performing such analysis. The number of control samples should be sufficient to carry out at least two complete analytical control product series in accordance with the requirements established during state registration. Storage of control samples of finished products and pharmaceutical substances should be carried out in accordance with the requirements of regulatory legal acts of the Russian Federation. Storage conditions must comply with the requirements established during the state registration of the medicinal product (for example, storage at low temperature, if required).
A control sample is a sample taken from a batch of raw materials, packaging material or finished products, which is stored for analysis during the expiration date of the batch, if necessary. Samples taken at critical intermediate stages (for example, after which analytical studies and release approvals are expected) and samples of intermediates that are shipped outside the manufacturer's control area should be retained, if the stability of the samples allows this. Designed for analysis and should be readily available to a laboratory that has validated methods for performing such analysis. The number of control samples should be sufficient to carry out at least two complete analytical control product series in accordance with the requirements established during state registration. Storage of control samples of finished products and pharmaceutical substances should be carried out in accordance with the requirements of regulatory legal acts of the Russian Federation. Storage conditions must comply with the requirements established during the state registration of the medicinal product (for example, storage at low temperature, if required).
 Archival sample - a sample in the final package, selected from a series of finished products, which is stored for the purpose of confirming identity. For example, during the shelf life of a batch, it may be necessary to inspect the sample or packaging, labeling, instructions for use, obtaining information about the batch number and expiration date. Archival samples must represent a series of finished medicinal products in the form in which they are sold in the Russian Federation and can be used for control in order to confirm compliance with the requirements established during state registration. Archival samples are recommended to be stored at the site where the authorized person who issued the permit for the release of products is located.
Archival sample - a sample in the final package, selected from a series of finished products, which is stored for the purpose of confirming identity. For example, during the shelf life of a batch, it may be necessary to inspect the sample or packaging, labeling, instructions for use, obtaining information about the batch number and expiration date. Archival samples must represent a series of finished medicinal products in the form in which they are sold in the Russian Federation and can be used for control in order to confirm compliance with the requirements established during state registration. Archival samples are recommended to be stored at the site where the authorized person who issued the permit for the release of products is located.
 In many cases, control and archival samples of finished products are identical and are units of production in the final package. In such cases, control and archival samples can be considered as interchangeable. Control and archival samples characterize a series of finished products or raw materials and materials, are an attachment to the dossier for a series and can be evaluated in the event of claims for the quality of a medicinal product, verification of compliance with the requirements established during state registration, verification of labeling and packaging, or verification by an authorized federal executive authority (inspectorate).
In many cases, control and archival samples of finished products are identical and are units of production in the final package. In such cases, control and archival samples can be considered as interchangeable. Control and archival samples characterize a series of finished products or raw materials and materials, are an attachment to the dossier for a series and can be evaluated in the event of claims for the quality of a medicinal product, verification of compliance with the requirements established during state registration, verification of labeling and packaging, or verification by an authorized federal executive authority (inspectorate).
 The manufacturer, importer or the enterprise where the permission to release the batch is issued must keep control and (or) archival samples of each batch of finished products, the manufacturer - control samples of each batch of raw materials and (or) intermediate products. On each production site packaging company must keep control samples of each batch of primary packaging materials and printed materials. It is allowed to include printed materials in the composition of control and (or) archival samples of finished products.
The manufacturer, importer or the enterprise where the permission to release the batch is issued must keep control and (or) archival samples of each batch of finished products, the manufacturer - control samples of each batch of raw materials and (or) intermediate products. On each production site packaging company must keep control samples of each batch of primary packaging materials and printed materials. It is allowed to include printed materials in the composition of control and (or) archival samples of finished products.
 Control and archive samples of each batch of finished products must be stored for at least the expiration date of the batch and one year after the expiration date. The control sample must be packed in its original packaging. Samples of raw materials (except for solvents, gases or water intended for technological purposes) must be stored for at least two years after the release of the medicinal product, unless a longer period is provided for by the relevant regulatory legal acts of the Russian Federation.
Control and archive samples of each batch of finished products must be stored for at least the expiration date of the batch and one year after the expiration date. The control sample must be packed in its original packaging. Samples of raw materials (except for solvents, gases or water intended for technological purposes) must be stored for at least two years after the release of the medicinal product, unless a longer period is provided for by the relevant regulatory legal acts of the Russian Federation.
The objects of standardization in the field of drug circulation are medicines and activities related to:
organization of control of production and quality of medicines;
the process of organizing the provision of medicines at the federal and regional levels;
the manufacture of medicines by pharmacies;
processes occurring in the commodity distribution network;
drug information for consumers;
drug supply in the health care facility system;
rational use of medicines, as well as the activities of pharmacies.
The organization of production and quality control of medicines is regulated by the Industry Standard OST 42-510-98 - these are the Rules for the organization of production and quality control of medicines (GMP) (approved by the Ministry of Health of the Russian Federation on February 25, 1998).
The document is a set of rules and requirements for the organization of production and quality control of medicines for medical purposes. The main provisions also apply to the last stages of the production of medicinal substances intended for the manufacture of finished medicinal products. In full, the requirements for the production of medicinal substances, as well as certain groups of medicinal products, are set out in special regulatory documents. The rules are mandatory for all drug manufacturers, regardless of their departmental subordination or form of ownership.
Standardization of drugs is the main guarantor of their high quality in mass production and ensures the effectiveness and safety of use. The basis of the quality management system for medicinal products is standardization.
Certification of medicinal raw materials and products is carried out in accordance with the rules for certification in the Medicines Certification System of the GOST RF Certification System. These Rules define the basic principles and requirements related to the procedure for certification of registered in the territory Russian Federation medicines of domestic and foreign production in order to protect the rights and interests of consumers and to pursue a unified state policy in the field of providing the population with high-quality and safe medicines:
I. Procedure for certification of domestic and foreign medicines
II. Requirements for certification bodies
III. Requirements for control laboratories
IV. Maintenance of the State Register of Participants in the Certification of Medicinal Products
Creation regulatory framework in this area, it implements the tasks of providing the population with safe, effective and high-quality medicines. Drug provision consists in the development, testing, registration, production and sale of medicines.
Requirements for the development of new drugs include the regulation of drug development technology, their preclinical and clinical trials, registration rules.
Requirements for the sale of medicinal products regulate the conditions of storage, transportation, certification, wholesale and retail, supply of medicines to medical institutions, issuance to patients. An example is the order of the Moscow Health Department No. 257 dated May 25, 2004. on the procedure for the acquisition, transportation, storage, accounting, dispensing, use of narcotic drugs, psychotropic substances included in List II, and psychotropic substances included in List III, in accordance with the federal law "On drugs and psychotropic substances» in medical and preventive institutions of the Moscow Department of Health.
State centralized system drug provision in the USSR provided a certain level of pharmaceutical assistance to the population through strict administration. Due to the presence of a vertical management of the drug supply system and the homogeneity of wholesale and retail Drugs quality of drug supply could be regulated by orders, instructions and circular letters. At the same time, the lack of flexibility characteristic of planned regulation had a negative impact on the quality of providing the population with drugs.
feature modern stage development of the pharmaceutical market should be considered the emergence of pharmacy chains, formed, as a rule, wholesalers various forms of ownership or, much less frequently, by Russian manufacturers.
Since drug supply is an integral part of the entire sphere of drug circulation and is inextricably linked with the state quality control system, standardization issues should be addressed together with the improvement of the drug quality control system.
Medicinal assistance to citizens is carried out on the basis of the requirements for fulfillment medical services. The formation of regulatory documents that allow organizing the provision of medicines is based on the minimum requirements of the protocols for the diagnosis and treatment of diseases. These documents include:
List of vital and essential medicines.
Federal Guidelines for Physicians on the Use of Medicines.
List of preferential release of medicines.
List of medicines available without a doctor's prescription.
Formulary list of medicines of the subject of the Russian Federation.
Formulary list of health care institutions.
Mandatory assortment of medicines for pharmacies serving outpatients.
An important issue is to ensure the quality of information on medicines. To solve this problem, the Industry Standard 91500.05.0002-2001 "State Information Standard of the Medicinal Product. Basic Provisions" (OST GISLS) was developed and implemented.
The state information standard of a medicinal product includes the following structural elements:
- - pharmacopoeial article of the medicinal product;
- - Formulary entry of the medicinal product;
- - clinical and pharmacological article;
- - passport of the medicinal product.
Approved official registration documents identifying the medicinal product:
1. Registration certificate - a regulatory document confirming the fact of official permission for the circulation of this medicinal product on the territory of the Russian Federation.
Contains information on the registration of a medicinal product for the following items:
- - registration number;
- - date of registration;
- - trade name;
- - INN or a name replacing it in the prescribed manner;
- - enterprise - manufacturer;
- - country of the enterprise - manufacturer;
- - dosage form;
- - dosage;
- - FSP number.
Registration certificate - serves as the basis for the inclusion of a medicinal product in State Register medicines.
2. Evidence of registration of the unique packaging number of the medicinal product - digital designation(code) in the standard of the International Association of Commodity Numbering EAN (UNISCAN), which has an appropriate graphical representation (barcode), allows for its automated reading and unambiguous identification. A 13-bit digital code - in the form of a combination of strokes and spaces of different widths - includes codes: country, company that coded the product, the product itself and the control number.
Regulatory documents regulating the sphere of circulation of medicinal products are developed on the basis of information contained in the state information standard of the medicinal product. Compliance with GISLS is a criterion for the reliability of information about a medicinal product.
Based on the information contained in the GISLS, the following regulations:
State Register of Medicinal Products - a systematized list of names and main characteristics of medicinal products, medicinal products approved for use in the Russian Federation;
Instructions for use of a medicinal product - an official document containing information about a medicinal product that is necessary and sufficient for its effective and safe medical use;
List of vital and essential medicines - a systematic list of generic names of medicines, including drugs, without the use of which, according to experts, in life-threatening diseases and syndromes, the disease will progress or its course will worsen, complications or death of the patient will occur, as well as medicines for specific therapy of socially significant diseases;
Federal guidelines for physicians on the use of medicines - a collection of formulary articles of medicines or their fragments included in the list of vital and essential medicines with a description of the schemes and features of their use in a specific disease (syndrome);
List of preferential dispensing of medicines - a document approved by the local executive authority of a constituent entity of the Russian Federation, containing a guaranteed range of concessionary medicines for outpatient treatment;
List of medicines dispensed without a doctor's prescription - a regulatory document containing a list of names of medicines dispensed without a doctor's prescription;
Formulary list of medicines of a constituent entity of the Russian Federation - a document containing a list of names of medicines recommended for use in the territory of a constituent entity of the Russian Federation;
Formulary list of a healthcare institution - a document containing a list of names of medicines recommended for use in a healthcare institution;
Mandatory range of medicines for pharmacies serving outpatients - a regulatory document regulating the minimum range of medicines required to provide medical care, the presence of which is mandatory for a pharmacy organization serving outpatients.
For systematic work on the creation of a system of regulatory documents in the field of drug supply and the introduction of standards into practice, it is necessary to coordinate standardization work both in the field of drug circulation and in healthcare in general. Specialized scientific institutions, the pharmaceutical and medical community should take an active part in this work.
Chapter 13 Standardization, standards structure and approval procedure, registration, licensing and certification
In OST 91500.05.001-2000. Pharmaceutical quality standards. General provisions a unified procedure for the development, presentation, execution, examination, approval, approval and designation of quality standards for medicines has been defined.
The scheme for implementing the requirements of the OST on ensuring state control of the quality of medicines is shown in fig. 13.1.
This OST also defines that the state quality standards for medicines bear the general name "Pharmacopoeia article" and come in the following categories:
General pharmacopoeial monograph (GPM)- includes a list of normalized indicators or test methods for a specific dosage form, a description of physical, physicochemical, chemical, biochemical, biological, microbiological methods for the analysis of medicines, requirements for the reagents used, titrated solutions, indicators.
Pharmacopoeia article (FS) is developed for a medicinal product under an international non-proprietary name, if it is available (for monocomponent medicinal products) and contains a mandatory list of indicators and quality control methods (taking into account its dosage form) that comply with the provisions of leading foreign pharmacopoeias.
The development of a pharmacopoeial monograph for an original (proprietary) medicinal product during the term of patent protection and its inclusion in the State Pharmacopoeia is possible only upon agreement with the developer of the medicinal product or is carried out after the expiration of the patent.
Rice. 13.1. Scheme for the implementation of the requirements of the OST to ensure state control of the quality of medicines (according to Bagirova V.L.)
Pharmacopoeia article for a medicinal product of a specific enterprise (FSP) includes a list of standardized indicators and quality control methods for medicines produced by a particular enterprise, taking into account the requirements of the State Pharmacopoeia. Moreover, the normalized indicators should not be lower than the requirements established by the State Pharmacopoeia. The validity period of the FSP is established taking into account the level of the technological process of a particular production of a medicinal product, but not more than 5 years.
General monographs and pharmacopoeial articles for individual medicinal products constitute State Pharmacopoeia (GF), which must be published by the relevant federal agency and republished every five years.
At present, the State Pharmacopoeia of the USSR XI edition is the national standard in Russia. Preparing for the release of the GF XII edition.
One of the main international standards for medicines is introduced in 1996. European Pharmacopoeia, which is designed to ensure the public health of the population by creating public standards that regulate the quality of medicines.
Members of WHO can use International Pharmacopoeia, which regulates acceptable standards for the potency, purity and quality of pharmaceutical products entering the international market. A distinctive feature of this standard from national pharmacopoeias is the use of methods developed on the basis of classical methods for quality control of drugs. This makes them available to developing countries as well. The methods are easy to implement and do not require large material costs.
13.1. Structure and content of standards for medicinal products and medicinal plant raw materials
As mentioned earlier, pharmacopoeial articles are the quality standards for substances, drugs and medicinal plant raw materials. The exception is medicinal plant raw materials (angro), for which it is historically customary to formulate quality standards in the form of GOST.
13.1.1 General requirements to the structure and content of pharmacopoeial monographs
The general requirements for the structure and content of pharmacopoeial monographs for substances, medicinal products and packaged medicinal plant raw materials are as follows:
1. The name of the product in Russian is given in the title of the standard.
In the name of the product, the first word should be the name of the active substance (in the nominative case) or the trade name (in the nominative case), followed by the name of the dosage form, dosage (concentration), volume. For example: Analgin tablets 0.5 g or Analgin injection 25%.
The list of the main sections of the FS and FSP and the sequence of their presentation is determined by the specific dosage form.
All product quality indicators contained in the FSP must be presented in a summary table (specification). The specification is a mandatory part of the FSP.
The numbering of sections of the OFS, FS and FSP is not indicated.
The presentation of the text should be brief, without repetition and should exclude the possibility of different interpretations.
Abbreviations of words in the text and captions under figures, diagrams and other illustrations are not allowed, with the exception of abbreviations used in the legislation of the Russian Federation.
In the text part of the standards, the requirements for the quality of goods are stated in an imperative form.
When presenting mandatory requirements, norms and methods in the text, the words must, should, must and derivatives from them.
Section headings are placed on the red line and are bold or underlined.
If the requirements, norms, methods, etc., applicable to the medicinal product are established in the State Pharmacopoeia, other state or industry standards, then instead of repeating their text, a link to the source should be given.
The presentation of the text about the substance used in the manufacture of the medicinal product must be accompanied by a reference to the regulatory document according to which it is produced.
When describing the composition of the medicinal product, the quantitative content of active active substances and the qualitative composition of excipients are indicated in the form of a list with reference to the relevant regulatory documentation containing the requirements for their quality.
In chapter " Description» establish organoleptic indicators appearance finished medicinal product (color, smell). The definition should not be used: azure, egg, etc. The main color is placed at the end of the definition.
In chapter "Disintegration" the time of complete disintegration of a tablet or capsule in a liquid medium is indicated (under the conditions given in the State Pharmacopoeia).
Chapter " Authenticity» contains methods for determining the authenticity of the active substance and the product as a whole. For preparations of complex composition, after the description of the necessary definition, the identified ingredient is indicated in brackets.
Sections "Transparency" and " Chroma» establish the transparency (turbidity) and color of the product in comparison with the solvent or the corresponding standard.
Chapter " acidity, alkalinity" or "pH" regulates these indicators.
Sections"Dry Residue", "Alcohol Content", "Boiling Point", "Density", "Refractive Index", "Angle of Rotation", "Viscosity" the upper and lower limits of these standard indicators are indicated in the corresponding units of measurement.
Chapter " Dissolution" determines the amount of the active substance, which, under given conditions, must go into solution for a certain time.
In chapter " Quantitation» describes the method for the quantitative determination of the main substance contained in the medicinal product. This section also indicates the percentage of the main substance or activity in action units (U / mg) or micrograms per milligram (µg / mg) in terms of the active substance in the medicinal product or its dosage forms.
For tablets, the limits of the content of the main substance in grams in one tablet are indicated, counting on the average weight of the tablet, in suppositories - in grams per candle, in pills - in grams per pill, in injection solutions - in grams per 1 ml.
However, the structure and content of PS and PhSP for each type of pharmaceutical product has its own characteristics. Since in their activities the pharmacist, chief and senior nurses must be able to use these standards when accepting goods, and students of the Faculty of Higher Nursing Education do not study or work with these regulatory documents in any course, we will dwell on their structure and content in more detail.
13.12. Structure and content of state quality standards for substances
A pharmacopoeial monograph for a substance has the following structure and content:
The name of the substance in Russian
International nonproprietary name (INN) in Russian
Chemical name in accordance with the requirements of the International Union for Pure and Applied Chemistry - IUPAC).
Structural and empirical formulas.
Relative molecular weight.
Description (indicators of the appearance of the substance: physical state, color, smell, hygroscopicity, light and gas sensitivity).
Authenticity (characteristics of UV and IR absorption spectra, specific qualitative reactions, etc.).
Solubility (indicators of solubility in water, 95% alcohol, chloroform, ether, other solvents, the ratio of the mass of the solute and the volume of the solvent).
Temperatures of melting, solidification, boiling, decomposition.
Density.
specific rotation.
Specific absorption rate.
refractive index.
Transparency and color of the solution (indicated for solutions of a certain concentration; for colored solutions, the number of the color standard and the letter of the scale, the characteristics of the absorption spectra of the solutions are indicated).
Acidity and alkalinity (pH is determined by a potentiometric method using 0.01-ONE acids or alkalis using appropriate indicators).
mechanical inclusions.
Foreign impurities, purity indicators, residual organic solvents, sulphated ash, heavy metals, arsenic (detection methods, permissible levels of impurities that enter the substance during its production or are formed during storage).
Loss on drying, water (method of conducting a study according to K. Fischer, norms for loss in mass on drying or moisture content).
Toxicity, pyrogenicity, content of histamine-like substances (test doses, methods of administration and observation period for the tested substances).
Microbiological purity, sterility (method for determining microorganisms, their permissible limits).
Quantitative determination of the main active substance (its% content or activity in units / mg).
Packaging (type of primary packaging, number of product units in primary packaging, secondary packaging, number of primary packages in it, sealing methods; links are provided to regulatory documents that determine the types and quality of group and transport packaging, etc.).
Labeling (in accordance with the requirements for the graphic design of medicines).
Storage (product storage conditions, if necessary, requirements for protecting products from the influence of climatic factors are indicated).
Shelf life (the period during which the substance can be used).
Pharmacological, biological action (pharmacological group to which the substance is assigned).
Precautionary measures.
The name of the substance.
Introductory part.
Main part.
At the same time, clauses 1.1 - 1.3, 2.1, 2.3, 3.1-3.3, 3.13, 3.15-3.22 are mandatory. The inclusion of other items depends on the nature of the medicinal substance (substance), the technology for its production and dosage forms that will be made from this substance.
13.1.3. Structure and content of state quality standards for medicines
A pharmacopoeial article for medicinal products has the following structure and content:
The chemical name of the active substance (for packaged medicinal herbal raw materials - the Russian and Latin names of herbal raw materials; for extracts - the plant from which the raw material is made and its family.
The composition of the medicinal product, indicating the quantitative content of active active substances.
The composition of excipients (qualitative).
Description (organoleptic indicators of the appearance of the medicinal product).
Authenticity (characteristics of UV and IR absorption spectra, specific qualitative reactions, etc.).
Disintegration (time of complete disintegration of a tablet, capsule in a liquid medium).
Transparency, color (transparency, turbidity, color of the drug compared to the solvent or standard).
Acidity, alkalinity (pH, determined potentiometrically, using 0.01-0.1 N acids or alkalis, using appropriate indicators).
Dry residue, alcohol content, boiling point, density, refractive index, specific rotation, viscosity (upper and lower limits of the listed indicators).
Dissolution (the amount of the active substance, which must, under appropriate conditions and over a certain time, go into solution).
Quantitative determination (method of quantitative determination of the main substance contained in the medicinal product,% content of the main substance or activity in U / mg, μg / mg in terms of the active substance; limiting content of the main substance: for tablets - in grams in one tablet, for suppositories - in grams per 1 suppository, for pills - in grams per 1 pill, in solutions for injection - in grams per 1 ml).
Microbiological purity (method for determining microorganisms, their permissible limits).
Packaging (type of primary packaging, number of product units in primary packaging, secondary packaging, number of primary packages in it, sealing methods; references are given to regulatory documents that determine the types and quality of group and transport packaging, etc.).
Marking (given in accordance with the requirements for graphic design medicines).
Shelf life (the period during which the drug can be used).
Pharmacological action (pharmacological group to which the medicinal product belongs).
The name of the medicinal product in Russian.
Introductory part.
Main part.
13.1.4. Structure and content of standards for medicinal plant materials (angro)
GOST for medicinal plant materials (angro) has the following structure and content:
The name of the medicinal plant material in Russian and Latin.
Introductory part.
Includes the name and scope of the medicinal plant material, producing plant and family (in Russian and Latin).
External signs ( short description morphological features of whole and crushed raw materials).
Microscopy (description of diagnostic features of raw materials);
Qualitative reactions (methods of qualitative chemical reactions or chromatographic samples).
Quantitative indicators (rates of the percentage of active substances, biological activity, moisture rates, total and insoluble ash in 10% hydrochloric acid solution, admissible impurities and fineness).
Quantification (methods for determining the content of active substances).
Microbiological purity (method for determining microorganisms, their permissible limits).
Packaging (type of primary packaging, number of product units in primary packaging, secondary packaging, number of primary packages in it, sealing methods; references are given to regulatory documents that determine the types and quality of group and transport packaging, etc.).
Labeling (provided in accordance with the requirements for the graphic design of medicines).
Storage (product storage conditions, if necessary, requirements for protecting products from the influence of climatic factors are indicated).
Shelf life (the period during which medicinal plant materials can be used).
Pharmacological action (pharmacological group to which medicinal plant materials are assigned).
Main part.
For medicines, similarly to medical products, there are different kinds confirmation of product quality to the requirements of the State Standard, these include conformity assessment, which includes: conformity assessment, registration, accreditation, control and supervision etc.
Three groups of persons are involved in the conformity assessment, which represent two interested parties (for example, the supplier and the buyer) and an independent one - a person or body acting as an arbitrator.
The main activities in conformity assessment are confirmation of compliance of products or other objects, production processes, operation, storage, transportation, sale and disposal, performance of work or provision of services with the requirements of technical regulations, provisions of standards or terms of contracts.
Confirmation of conformity may be voluntary or mandatory. Mandatory confirmation is carried out in the form of acceptance of a declaration of conformity and mandatory certification.
13.3. Registration of medicines
In accordance with Art. I9 of the Federal Law No. 86-FZ of 06/22/98 "On Medicines" in Russia, only those medicines that are approved for use and registered in accordance with the rules No. 10 / 29-14 of 12/01/98, defining uniform requirements, can be used to the documentation for domestic and foreign drugs, the procedure and terms of their registration. In accordance with this law and the rules of registration, the following are subject to registration:
new medicines;
new combinations of previously registered medicinal products;
medicinal products previously registered but produced in other dosage forms, with a new dosage or other composition of excipients;
generic drugs.
At the same time, the procedure for accelerated registration is provided for the latter.
State registration of a medicinal product is carried out by the Federal Authority for Quality Control of Medicines within a period not exceeding 6 months (excluding the period of clinical trials) from the date of filing an application for state registration.
The applicant may be an organization developing a drug or another entity on behalf of the developer.
Registered drugs are entered in the State Register of Medicines.
Validity of the certificate of registration is 5 years with subsequent possible re-registration.
The procedure for registering medicines in the Russian Federation is a very expensive and rather lengthy process, especially when registering imported original medicines, and includes:
Preliminary examination of the registration dossier.
Registration dossier preparation:
translation of a number of official, instructive and normative-technical documents into Russian;
preparation of documentation for quality control and stability of the drug (final product control) in accordance with the Russian Pharmacopoeia and national standards;
reproduction of materials for the dossier in the required quantities.
Submission of the dossier to Federal Service on supervision in the field of health and social development.
Examination (with the participation of specialized commissions) of materials in terms of the safety and effectiveness of the presented medicine, the possibility of its registration in Russia.
6. Obtaining permits for implementation.
13.4. Licensing of activities for the production and sale of medical and pharmaceutical products
In accordance with Art. 15 of the Federal Law No. 86-FZ of June 22, 1998 "On Medicines" and federal law No. 128 of 08.08.01 “On Licensing Certain Types of Activities” all pharmaceutical companies engaged in the production or circulation of medicines on the domestic market, regardless of their form of ownership, are subject to licensing.
Issuance of a federal license for wholesale trade and manufacturing licenses for the J1C are administered by federal-level licensing commissions. However, due to the large number of pharmaceutical market entities in our country, the Ministry of Health of the Russian Federation also used its legislative rights in letters dated July 16, 2002 No. 2510/7125-02-32 “On the transfer of powers to license pharmaceutical activities” and No. 02-32 "On the transfer of part of the powers for licensing activities related to the circulation of narcotic and psychotropic drugs" transferred the issuance of territorial licenses for wholesale and retail trade to local (territorial) licensing commissions.
Companies that obtain a license to manufacture medicines are subject to the highest requirements.
Since 1994, the Ministry of Health of Russia has issued more than 500 licenses for the production of medicines to enterprises and institutions of various organizational and legal forms and departmental affiliation (for comparison, until 1992, only about 200 plants and factories carried out such activities in the entire USSR)
Documents submitted to the licensing commission include:
information about the manufacturer; a list of medicines to be produced; various technological information.
When making a decision to issue (or refuse to issue) a license, special attention is paid to the compliance of the technical level of production with the modern one, and for new production sites it is mandatory to comply with the industry standard OST 42-510-98 “Rules for the organization of production and quality control of medicines (GMP) ".
The issued license for the production of medicines is valid throughout Russia. Its validity period is determined by the licensing commission, but cannot exceed 5 years. The costs of the licensing commission for the consideration and issuance of a license, as well as for the related examination, are paid by the company that applied for the issuance of a license.
Licensing of pharmaceutical activities in the field of wholesale trade can be carried out in two versions: with the issuance of territorial and federal licenses. The first of them gives the right to conduct wholesale trade only in the territory that issued the license.
More stringent requirements are imposed when issuing a federal license that allows wholesale trade (as well as related activities, such as obtaining, storing medicines) throughout Russia. A federal license is issued only to firms that have received a territorial license for this type of pharmaceutical activity and have worked for them for at least 6 months.
The federal license is issued by the licensing commission of the Ministry of Health and Social Development of the Russian Federation. Among the documents required to obtain it, a copy of the agreement on the quality control of medicines with accredited control and analytical bodies is provided; certificate of participation in the implementation of territorial medical programs to provide medicines to territories and regions; characteristics of the company's activities issued by the territorial licensing commission; indicators of the volume of performed activities. Thus, the licensing commission of the Ministry of Health gets the opportunity to make sure that the company has a good reputation in its territory and, due to the nature of its activity, objectively needs to obtain a federal license.
Licensing of pharmaceutical activities of pharmacies is a way of state control over compliance with the requirements of the legislation in the field of pharmaceutical activities related to the sale of medicines. The activity of a pharmacy institution of any form of ownership is prohibited without a State license. Licensing of the activities of pharmacies is carried out in strict accordance with the current legislation, as well as the instruction of the Ministry of Health of the Russian Federation dated 12/14/92 "On the procedure for licensing the pharmaceutical activities of pharmacies and pharmacies in the Russian Federation".
To obtain a license, a pharmacy institution applies to the licensing commission operating under state administration bodies, city and district local administrations. Thus, the issuance of licenses in Moscow is carried out in the Moscow Licensing Chamber. To obtain a state license, a pharmacy institution submits the following documents to the licensing commission:
Statement.
A copy of the certificate of state registration of the institution (enterprise), certified by a notary.
A copy of the Charter or Regulations of the licensed institution, approved in the prescribed manner (notarized).
A copy of the order or contract for renting a room, equipment (notarized).
List of types of pharmaceutical activity, services declared for licensing.
The conclusion of the state sanitary supervision.
The conclusion of the state fire supervision.
Accreditation certificates of the pharmacy institution and staff.
A copy of the previously issued license (for an institution that has previously passed licensing).
A copy of the payment order for the payment of a one-time fee for the issuance of a license.
The licensing procedure is determined by the commission and provides for:
1) study of the documents submitted by the pharmacy;
conducting an on-site examination;
issuance of a license.
If the documents submitted by the pharmacy do not meet the requirements, they are returned to the applicant. The new term for consideration is calculated from the moment of re-submission of documents.
Licensing is subject to each type of pharmaceutical activity performed by the company applying for a license, namely:
production of all types of dosage forms according to the prescriptions of doctors and the requirements of medical institutions;
production of all types of dosage forms according to frequently repeated prescriptions of doctors in small batches;
control over the technology of manufacturing, storage, quality of finished medicines and medicines manufactured in pharmacies, medicinal plant materials and preparations from it;
receipt, storage, organization, delivery, dispensing of medicines and medical devices to pharmacies and medical institutions;
sale to the population and medical institutions of medicines (both manufactured in pharmacies and ready-made) and medical products approved for use in the Russian Federation.
Before obtaining a license, a pharmacy institution and individuals engaged in individual or collective activities in the drug supply system must undergo accreditation, which consists in determining the compliance of the conditions and place of their activities with the established requirements for pharmacies.
13.5. Certification of medicines
The drug certification system operates within the framework of the mandatory certification system established by the State Standard of the Russian Federation and currently headed by the Federal Agency for Technical Regulation and Metrology.
Legal basis of the system of certification of medical and pharmaceutical products includes the following legal acts and orders:
1. Fundamentals of the legislation of the Russian Federation on the protection of the health of citizens.
Decree of the Government of the Russian Federation "On Licensing certain types activity” (dated 24. 12. 94 No. 1418).
Regulations on the Ministry of Health of the Russian Federation (approved by the Decree of the Government of the Russian Federation dated 03.06.97 No. 659).
Documents approved by the Ministry of Health of the Russian Federation:
Rules for conducting certification in the Russian Federation.
Amendment No. 1 of the "Rules for Certification in the Russian Federation".
The procedure for conducting certification in the Russian Federation.
Change No. 1 "Procedure for certification in the Russian Federation".
Rules for the use of the conformity mark for mandatory product certification, etc.
GOST R 40.001-95. Rules for the certification of quality systems in the Russian Federation.
GOST R 51000.1-95. State system standardization of the Russian Federation. The system of accreditation of certification bodies and testing and measuring laboratories. General requirements.
GOST R ISO/IEC 65-2000. General requirements for product certification, etc.
Mandatory and voluntary certification is carried out according to the schemes described above (see Chapter 11, Table 11.4) and represents a given set of actions officially accepted as evidence of compliance of medicines with the requirements of the technical regulation and standard. Depending on the task of certification, a scheme is selected and verification is carried out according to it.
The validity period of the certificate of conformity for the medicinal product corresponds to the expiration date. In the accompanying documentation attached to the certified products, a record is made of the certification, which indicates: the number of the certificate, the validity period, the body that issued it.
The procedure for certification of medicines is as follows: 1. The applicant submits an application to the Certification Body. The following documents are attached to the application:
registration certificate of the Ministry of Health of Russia with permission to use J1C in medical practice;
license for the right to manufacture (sale) J1C;
an act of taking an average sample (taking an average sample is carried out in accordance with the requirements of the article “Sampling (sampling) of medicines” of the State Pharmacopoeia);
manufacturer's analysis protocol (for domestic J1Cs) or a company's analysis certificate and its translation (for foreign drugs) with the results of drug quality checks for compliance with ND requirements upon release.
The applicant may be a legal entity of any form of ownership (or an individual) that has obtained, in accordance with the established procedure, a license to manufacture, sell or import medicines into the territory of the Russian Federation.
Currently, throughout the Russian Federation, there are certificates of conformity issued by:
Center for Certification of Medicinal Products of the Ministry of Health of Russia;
Center for Certification and Quality Control of Medicinal Products in Moscow;
State Health Institution "North-Western Center for Quality Control and Certification of Medicinal Products", St. Petersburg. A sample of filling out a certificate of conformity for a sample medicinal product until April 6, 2004 is shown in fig. 13.2.
13.6. Ecological aspects of drug merchandising
Ecology- is the science of the relationship of plant and animal organisms and their communities with each other and environment. The term was proposed by Haeckel in 1886. Nowadays, ecology has acquired special significance as the scientific basis for the rational use of natural resources and the protection of living organisms.
Since the 1970s, human ecology, or social ecology, has been developing, studying the patterns of interaction between society and the environment, as well as the practical problems of its protection. This is of interest:
1. Study of the anthropogenic impact of the external environment on the product:
contamination of raw materials and materials (basic stage);
pollution during technological production(technological stage);
pollution during transportation and storage (handling stage);
Sksgsna gotnkkavpk GOST R. MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION
DEPLGGLMSHT VESSEL(1""1"QC.>"FYa"MVN<К.Ш, Г.КЮНАСИОСГИ ЛЕКАРСТВЕННЫХ (РЬДСГ» II МЕДИМШСКОЙ ТЕХНИКИ
"m -Yes -cast g os blows tvesh yue "our 1 certification minsh gersgod zd?abs;phgsh;tsh
RUSSIAN FEDERATION"
Rice. 13.2. A sample of filling out a certificate for a medicinal product.
CERTIFICATE OF CONFORMITY
" 16 "" iWII.
Fnig Yaymk JAO - SIL yutgtSHINP YATIA"
"K" shmyay lpyauien? yAfltroscpai:j. h> jaekgrsgyeinm means, „
"-N^YASHSHSH
grkk goyaiosgi 19.W0S
j tilemkn W JU, N .itf pinuangle
".O ^ shtstvokg tloyliiyay SHI" MATNCHLOPG. 1 DSMSUMENTL
Itf-" IWS .....
C^JWjf.j^gaf"iJ" tfm m inis* MP. Tsshmyuiya Russian
<":::Щ"|)IR;1GKijffin TtaiXiiuB^BHH
j^ftirt- tutft/rtorfsm:*YY fv" 17 GJ _
Cpu*jarteTMW«pt»|ai™iir,TO ^..-"f " Zkamga 2В£Yah.

Kv tUkCKi K j!
contamination during use (operational phase).
2. Study of the impact of the product on the ecosystem and humans
pollution by industrial products;
packaging contamination;
the impact of the product itself.
The term pollution is understood as the introduction into any environment (atmosphere, water, soil, raw materials, etc.) of new, unfavorable, unfavorable agents or the excess of these agents in the environment from the natural long-term average level.
Pollution happens chemical(gaseous, solid, liquid and other agents); physical(thermal, electromagnetic, radiation, etc.); mechanical(contamination with packaging elements); biological(pollution by microorganisms, insects, etc.).
In 1989, at the pharmaceutical faculty of the MMA named after. I.M. Sechenov, the Scientific and Methodological Center for Microelement Analysis of Medicinal Products and Plant Raw Materials was established. About 80% of species of medicinal plants have been studied for the content of heavy metals.
traps using the most modern methods: atomic absorption spectroscopy, atomic emission spectroscopy, X-ray fluorescence spectroscopy, etc.
In June 1990, the First International Scientific Symposium "Environmental Aspects in Pharmacy" was held, at which a resolution was adopted on the creation of the state program "Environmental Safety of Medicines", including the creation of environmentally friendly technologies, complex processing of raw materials, development of work on biotechnology, identification and regulation of compounds of anthropogenic origin in natural medicinal raw materials, improvement and unification of methods and control systems, creation of databases and regional information systems. Since 2000, a new course "Ecology of Medicines" has been introduced at the pharmaceutical faculties.
Questions for self-control (module 4)
How important is the quality of medical and pharmaceutical products?
What types of quality indicators do you know?
What documents regulate the quality of medical and pharmaceutical products?
What is the significance of standardization as a factor influencing the formation of the use value of pharmaceutical products?
What are the goals and objectives of standardization?
What standards are used in the pharmacy network for working with pharmaceutical products, with the exception of medicines? Give them a description.
What standards are used for working with medicines? Give them a description.
Why does certification affect the use value of goods?
What is the meaning of certification and why is it needed in pharmaceutical practice?
What documents govern the certification of medicines?
What is the purpose of licensing medical and pharmaceutical activities?
What are the similarities and differences between GOST for medical devices and FS for medicines?
BASICS OF COMMODITY ANALYSIS
STANDARDIZATION(eng. standart norm, sample, measure) - the process of establishing and applying standards, i.e., a set of normative and technical documents regulating a set of norms, rules, requirements for the object of standardization, approved by the competent authority. S. is carried out in relation to both material objects (industrial and agricultural products, standards, samples of substances), and non-material (norms, rules and requirements for various purposes). The object of S. are also various forms of services, methods and means of ensuring the unity and accuracy of measurement, terminology, standard forms of documents, symbols, environmental protection and rational nature management, etc. A special scope of S. is medicine, in a swarm medicines, other products honey serve as S.'s objects. destination, labor safety (see below).
A typical example from the region of S. can serve as the so-called. standard samples, i.e., substances that have fairly accurately known and officially certified values of properties (parameters) specific to a given substance. According to their purpose, they belong to the class of metrological means (see Metrology). They are made according to a special technology, and the values of properties (parameters) are established based on the results of studies carried out according to a given program.
Reference materials are widely used in the extraction and processing of mineral raw materials, in the control of raw materials and materials supplied within the framework of international economic cooperation, in healthcare (for the unification of clinical and biochemical analyses), in the field of environmental protection (surveillance and control of environmental pollution), etc. d.
In the USSR, standardization is one of the elements of the state technical policy, closely connected with the system of planning and management of the national economy. The main task of S. in this case is the establishment of uniform requirements for the technical level and quality of products, raw materials, materials, components, as well as the standardization of requirements and methods for the design and manufacture of products for one purpose or another. The use of standards on a national scale helps to improve the quality of products, ensures an increase in the level of unification and interchangeability, creates the best opportunities for automating certain production processes and improving working conditions.
The state system of standards of the USSR unites work on S. at all levels of management of the national economy. It is a set of rules and regulations that unites the goals and objectives of S.; the organization and technique of carrying out S.; the procedure for the development, implementation and circulation of regulatory documents, as well as making changes to them; the procedure for state supervision and departmental control over their implementation and compliance; S. objects, categories and types of standards, etc. The planning of all activities in the S. area is an integral part of the state planning system.
The first document in the history of Soviet power in the field of measurement was the decree of the Council of People's Commissars of the RSFSR (1918) "On the introduction of the international metric system of measures and weights." The first all-Union standard was introduced in the USSR in 1926: OST-1 “Wheat. Selection varieties of grains. Nomenclature". In subsequent years, numerous standards were developed and approved, covering almost all branches of industry and agriculture in the Soviet Union. In 1978, there were already St. 20 thousand
A characteristic feature of S. in our country is the transition from individual S. to the development and implementation of intersectoral systems of standards of national importance. These include: the State Standardization System (SSS), the State Measurement System (GSP), the Unified System for Design Documentation (ESKD), the Unified System for Technological Documentation (ESTD), the Unified System for Technological Preparation of Production (ESTPP), the Unified System of Occupational Safety Standards (ESSBT) ), a system of standards for environmental protection, and so on. Programs are also being developed for integrated environmental protection for the most important types of products. They provide uniform requirements for the technical level and quality of products, components, raw materials, materials and equipment, for the means of control, measurement and testing of products, etc.
Since 1954, the State Committee of the USSR for Standards (Gosstandart) has been carrying out the general management of S. on a countrywide scale, and in the field of medicines, vaccines and sera - M3 of the USSR. The system of bodies and services of the USSR State Standard includes republican administrations, centers for S. and metrology, research institutes, and laboratories for state supervision of compliance with standards. St. 600 head, basic organizations of various branches of the national economy of the USSR carry out certain types of work related to standardization.
In addition to the State Standard of the USSR, M3 of the USSR also has the right to standardize in the field of medicine and health care. The Department for the Introduction of New Medicines and Medical Equipment M3 of the USSR carries out C. in relation to medicines, medical equipment, pharmacy equipment, medical biological and immunobiological preparations, as well as a number of other measures aimed at preserving human health.
The standards themselves, depending on the order of approval, as well as the scope of application, have several categories: state standards (GOST), industry (OST), republican (PCT), standards of enterprises (associations) - STP.
State standards (GOSTs) are approved by the USSR State Committee for Standards (Gosstandart), with the exception of certain types of standards approved by the USSR Council of Ministers, the USSR State Committee for Construction (Gosstroy) and M3 of the USSR.
GOSTs are obligatory for all ministries, state committees, departments of the USSR, for all state, cooperative and other public organizations.
Industry standards (OSTs) are approved by all-Union and Union-Republic ministries, state committees and departments of the USSR. They are mandatory for all enterprises, organizations and institutions of the department that approved the relevant standard. OSTs for products are approved by ministries or departments, which are the main ones in the design or production of this type of product, and are mandatory for other ministries (departments), enterprises, organizations and institutions associated with the production, development, transportation, storage, operation of the corresponding type products.
Republican standards (RST) are approved by the republican councils of ministers of the union republics (or, on their instructions, by the State Planning Committees or state construction departments of the union republics). PCT are obligatory for all enterprises, organizations, institutions located on the territory of the union republic, regardless of departmental subordination.
Standards of enterprises or associations (STP) are obligatory only for the enterprise (association), organization and institution that approved this type of standard.
On the basis of the relevant standards, technical specifications (TS) are developed, that is, a regulatory technical document (NTD) that establishes a set of requirements for a particular type of product.
In the C. process, standards are developed, approved, and modified by special state bodies in the manner prescribed by the State Standardization System. For each standard, the terms of entry into force are provided, and the scope of application is determined by the category of the standard (ie national, sectoral, republican, etc.).
A characteristic feature of S. is its technical and legal competence. This is typical for S. in relation to products, technological processes, environmental protection, labor safety, etc. The execution and control over compliance with standards is ensured by the relevant norms of the current law, which provides for legal liability for violation of the standard.
In the field of health care, there is a whole system of legislative acts (see Sanitary legislation), which ensures compliance with the standards aimed at preserving the health of the Soviet person (see Occupational health, Hygienic standards, Environmental protection, Labor protection and other similar articles) .
A characteristic feature of the present stage in the development of metrics in the USSR is the complexity of metrics. This is manifested in the fact that when certain standards are put into effect, the indicators of interrelated standards and the timing of their entry into force are provided for. The complexity of S. is ensured by the development of S. programs that combine products, semi-finished products and materials, technical means, the organization of production, and so on. The complexity of S. makes it possible to more effectively use the possibilities of modern production, to carry out intersectoral coordination, and to satisfy the requirements of interested parties.
Standardization within the framework of the CMEA is consistent with the tasks of the Comprehensive Program for the Further Deepening and Improvement of Cooperation and the Development of Socialist Economic Integration of the Member Countries of the Council for Mutual Economic Assistance (CMEA). S. in the CMEA are dealt with by the Standing Commission on S., the sectoral permanent commissions, the CMEA Institute for S. and the S. department of the CMEA secretariat. The main areas of work are the creation of systems of normative and technical documents (the system of normative and technical documentation of the CMEA for S.; the automated information and control system of S. and metrology of the CMEA; a unified system of design documentation of the CMEA; a unified system of tolerances and landings of the CMEA), as well as complex standards on products that are the subject of trade between the CMEA member countries. The norms and requirements of the CMEA standards correspond to international standards. By January 1, 1975, 4,900 CMEA recommendations on S. and 120 CMEA standards were adopted. The 28th CMEA Session (June 21, 1974) approved the "Regulations on the CMEA Standard (CMEA ST)" and approved the Convention on the Direct (Immediate) Application of CMEA Standards. The Convention on the Application of ST CMEA was ratified by the Presidium of the Supreme Soviet of the USSR by decree of September 17, 1974. The development and application of CMEA standards have a decisive influence on the intensification of the processes of socialist economic integration, the improvement of the international socialist division of labor, the increase in the level of production and product quality, and the strengthening of the competitiveness of socialist products. countries in the world markets and give a significant economic effect. The use of CMEA standards in the national economies of the CMEA member countries leads to a further convergence of national systems of accounting.
International standardization is associated with the development of multilateral scientific, technical and economic cooperation. In addition to national organizations, more than 300 international and regional organizations deal with questions of measurement, metrology, and improving the quality of products (1975). The largest international organizations operate in the S. area: the United Nations Economic Commission for Europe (UNECE), the International Organization for Standardization (ISO), and the International Electrotechnical Commission (IEC).
International standards and recommendations developed by these organizations establish indicators that meet modern scientific and technical requirements for quality, reliability, safety, and other important properties and characteristics of various types of products that are the subject of international trade, and also define unified methods and means of testing and certification materials and goods. The application of international standards contributes to the expansion of scientific, technical, economic and trade relations. International standards are widely used in the development of national standards, which can significantly reduce the time and cost of their development and get a great economic effect.
Standardization in medicine
Standardization in medicine is the development and use of uniform norms, rules, requirements in the development and implementation of medicinal, immune and other therapeutic, preventive and diagnostic agents, as well as medical equipment. C. contributes to the streamlining of these processes, taking into account requests and with the participation of all interested parties. C. is carried out taking into account the achievements of science, technology, best practices of medical, sanitary-epidemiological, research, pharmacy and other medical institutions, as well as enterprises of the medical and other industries that produce products for medical diagnostic and preventive purposes. S.'s results are reflected in normative and technical documents - standards that are mandatory for use, and some other documents of a similar purpose (norms, methods of their control, instructions, medical technical requirements, rules, regulatory materials, methodological recommendations and instructions) developed in in the prescribed manner and approved by the relevant departments of the M3 of the USSR.
Specifications - regulatory and technical documents (NTD) that establish a set of requirements for specific types, brands, articles of products. Specifications are an integral part of the complex of technical documentation for products, to which they apply. Specifications containing requirements related to ensuring labor safety and health protection of workers and employees are subject to agreement with the health authorities in the manner established by the Ministry of Health of the USSR.
Medical and technical requirements (technical specifications) are developed based on the study and analysis of the achievements of domestic and foreign technology, advanced production technology, the results of research work, as well as on the basis of the requirements of the customer - M3 of the USSR.
Medico-technical requirements (technical specifications for products of medical equipment and other medical products) are approved by the Office for the Introduction of New Medicines and Medical Equipment M3 of the USSR and the authority to which the developer's organization is subordinate.
S. is used in medicine in relation to medicines (see Pharmacopoeia, State Register, Medicines Control), pharmacy equipment (see Pharmacy), medical devices and medical equipment, such as: tools, devices, apparatus, patient care products , honey. equipment, etc. (see Medical equipment), honey. biological and immunobiological preparations (see Bacterial standard, Control of bacterial preparations), food (see Nutrition), special types of clothing (see Special clothing), personal protective equipment (see). In addition to material objects, S. covers in medicine the entire system of norms prescribing in the form of rules, letters of recommendation (methodological) requirements, etc. hygiene and organizational measures, clinical trials, laboratory research. This also includes the regulatory requirements for working conditions prescribed by sanitary rules (see Occupational health, Occupational safety, Maximum permissible concentrations, Safety precautions, Labor, Ergonomics) and labor standards associated with radiation or toxic hazards (see Radioactive substances, Radiation hygiene, Radiation safety, Toxicology), the state of air in domestic, sports and other premises, the quality of water, the purity of the soil, the size and quality of living space (see Communal hygiene), emissions from industrial enterprises (see Environmental protection) and all others norms that are essential for protecting public health, as well as requirements for compliance with these norms (see Hygienic standards, Sanitary inspection, Sanitary legislation, Sanitary and epidemiological station, Sanitary supervision). A special place is occupied by the standardization of measurement methods, measuring equipment and the units of measurement used (see Units of measurement, International system of units, Metrological health service, Metrology, Measuring instruments for medical purposes). The page extends also to honey. documentation (see Medical documentation) and honey. terminology (see Medical terminology).
The planning of work on standardization in the field of medicine belongs mainly to M3 of the USSR. All work on S. in the USSR M3 system is entrusted to the Administration for the Introduction of New Medicines and Medical Equipment M3 of the USSR. In addition, relevant specialists from the Main Directorates and Directorates of the M3 of the USSR take part in the work on S.: sanitary and epidemiological, pharmacy, medical and preventive care, medical and preventive care for children and mothers, quarantine infections, for the production of bacterial and viral preparations, the State Inspectorate for quality control of medicines and medical equipment.
Prepare standardization documents and standards, carry out standardization service in the M3 system of the USSR head (GOS) and base (BOS) organizations for standardization, research institutes performing standardization tasks, and departments of standardization and metrology of sanitary and epidemiological stations, head and base organization of the metrological health service (see Metrological health service).
Bibliography: Babayan E. A. and Utkin O. B. Basic provisions for approbation of medicines in the USSR and foreign countries, M., 1982; State system of standardization, M., 1975; State Pharmacopoeia of the Union of Soviet Socialist Republics, M., 1968; WHO Expert Committee on Specifications for Pharmaceutical Products, Ser. tech. report No. 645, Geneva, 1981; International Pharmacopoeia, vols. 1-2, Geneva, 1981; Methodological guide for laboratory assessment of the quality of bacterial and viral preparations, ed. S. G. Dzagurova and others, p. 5, M., 1972; All-Union classifier, Industrial and agricultural products, Higher classification groups, M., 1977; Bases of the legislation of the USSR and union republics about health care, M., 1970; System of labor safety standards, M., 1978; Sklyarov A. M. and Serebryany L. A. Requirements of the system of labor safety standards and their place in regulatory and technical documentation, Gig. labor and prof. ill., No. 12, p. 1, 1979; Standards, strains and methods of control of bacterial and viral preparations, ed. S. G. Dzagurova et al., M., 1979; Sumarokov A. A. Analysis of the scientific and methodological foundations of standardization of vaccines on the model of cholerogen-anatoxin, Zh. micr., epid. and immuno., No. I, p. 32, 1975; Sumarokov A.A., Salmin L.V. and Khlyabich G.N. Epidemiological assessment of the system of indicators characterizing the quality of vaccine preparations, ibid., No. 7, p. 78; Utyamyshev R. I. and Grishin A. N. Prospects for the development of an integrated quality management system for medical equipment in the healthcare sector, Standards and Quality, No. 1, p. 43, 1983.
E. A. Babayan, M. Ya. Kaabak, V. Ya. Maksimov; S. G. Dzagurov, A. A. Sumarokov (imm.), Yu. F. Krylov, V. D. Kucherenko (farm.), L. A. Potanina, L. A. Serebryany (gig.).
Introduction
Medicines (PM) - substances or a mixture of substances of natural or synthetic origin, which are used for the treatment, prevention and diagnosis of diseases. Poisonous and potent substances (arsenic, mercury, narcotic drugs and psychotropic substances) are often used as drugs, which, if overdosed, can lead to severe poisoning and even death. Among drugs, there are even dosage forms that are potentially hazardous to health, in particular, injections, in which even harmless substances become dangerous (mechanical inclusions), with completely unresolved problems of pyrogenicity and sterility. This specificity imposes stringent requirements on the standardization and methods of drug quality control, which should guarantee their safety and pharmacological action. In this regard, as well as with established traditions, standardization and quality control of drugs in all countries are usually carried out by a separate independent state structure.
General principles of the drug standardization system
Standardization of drugs- development and application of unified requirements and methods for the study of dosage forms (standards).
LS quality standard- a regulatory document containing a list of standardized indicators and methods for quality control of medicines, approved by the Ministry of Health of the Russian Federation (Ministry of Health of Russia).
The standardization system should impose such requirements for the development, clinical trials, production and sale of drugs that would ensure their maximum safety, the required pharmacological action and guaranteed quality at all stages of their use.
In general, such requirements are most fully reflected in the requirements of GLP (Good Laboratory Practice), GCP (Good Clinical Practice) and GMP (Good Manufacturing Practice), which are special cases of international standards ISO 9000 and regulate preclinical and clinical trials, production and sale of drugs . Note that USP XXIII also contains GMP requirements for drugs. Currently, domestic GMP requirements have been prepared in Ukraine.
The transition to the requirements of GLP, GCP and GMP requires a fairly high production culture and high material costs. In those countries where, for some reason, the conditions for a complete transition to these requirements have not yet been formed, their main elements are used when creating a drug standardization system.
Basic principles of drug standardization:
1. Only those drugs that are approved for medical use and sale in a given country can be sold.
standardization drug certification
2. Only those drugs for which there is an analytical regulatory and technical documentation (NTD) approved or agreed by the relevant state body can be sold.
3. NTD should provide objective quality control of drugs obtained using this technology. In the general case, a specific NTD can provide an objective control of a drug obtained only by a specific technology.
4. The level of production should ensure the possibility of obtaining drugs with quality indicators laid down in the NTD.
5. The quality control system for drugs sold should provide the possibility of detecting defects.
Paragraph 3 means that, in general, one and the same NTD cannot control drugs obtained using different technologies. For example, in some technological scheme for obtaining the substance of paracetamol, organic solvents are not used, therefore, in the NTD developed for this technology, they are not controlled. If a new technology for the production of paracetamol is being developed, which uses, for example, benzene, then it is obvious that its quality cannot be controlled according to the previous NTD. It is necessary to additionally introduce control of residual amounts of benzene. A similar situation arises with the control of technological impurities, which can be significantly different for different technologies. Therefore, the current NTD, in general, can control only the technology on the basis of which it was developed. Any changes in technology may require adjustment of the requirements of the NTD.
Unfortunately, the requirements of paragraph 3 are very often misunderstood and underestimated. A typical situation: an imported substance (paracetamol) was imported into Ukraine, the production technology of which was unknown, it was controlled according to the current NTD and, based on this, a conclusion was made about the quality of this substance. Obviously, this is not enough. Since the technology is unknown, then residual solvents, process impurities are unknown, which may not be controlled by the above NTD. If there is a need to import this substance, then additional (primarily chromatographic) studies should be carried out. The situation is similar with ready-made drugs.
Paragraph 4 means that not every pharmaceutical company can produce this drug. For example, the production of injections requires special requirements for cleanliness, equipment, and personnel. Otherwise, it is basically impossible to get a quality drug.
Item 5 characterizes the relationship between producers and consumers. The quality control system should ensure that manufacturers maintain an appropriate level of production, comply with the requirements of scientific and technical documentation and regulations, as well as the possibility of detecting accidental marriage and falsification. The latter is especially important for potent and vital drugs.







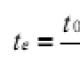


For beginners: breeding a broiler at home Boiled water for broilers
Only lovers will survive
Features of advertising aimed at children
retouching old photos in photoshop retouching old photos
What is an NPO: decoding, definition of goals, types of activities Does a non-profit organization have the right