Content
In colloquial language, new terms often appear, the sound of which for the opponent may seem unfamiliar, meaningless. It is not very convenient to ask the interlocutor again, and I would not want to demonstrate my incompetence in public either. Therefore, it is recommended to study new words of colloquial speech, find out their meaning.
What is validation
This is one of the thematic terms that is difficult for a simple layman to explain, it is even more difficult to understand the exact definition in scientific language. Primary sources provide an interpretation that is difficult to perceive, so it is better to use simple, accessible examples from life. So, there is the concept of validation - what is it in simple terms? After studying the scientific explanation, we can draw a conclusion. This unfamiliar word is close in meaning to such a concept as certification, which means a global check of the product according to all the parameters that were originally set by the customer.
For example, if we are talking about a mountain bike, this vehicle is considered validated if the customer rode it, while being satisfied with the speed, quality of work performed, functions, design and other parameters initially specified by the project. Simply put, this is a control test, so that the user can personally verify the result, a really profitable purchase.
Validation in general civil law
This word can mean the legal force of a document, often found in civil law. In simple terms, this is legalization, acceptance as a norm, approval. For example, a document after the expiration of the specified period enters into force, becomes valid in the legal sphere, jurisprudence. Yes, validation confirms final decision judges, and an appeal is no longer possible.
Validation in systems programming
In this area, the meaning of the word is associated with the receipt, processing, verification and transmission of data. Validation is relevant for any account user, as it confirms and officially proves the correctness of actions. To understand what this word means accessible language, can be brought good example about customer satisfaction:
- A person who is engaged in copywriting sells his articles.
- Before selling, he checks the data for errors and plagiarism using various systems in online mode.
- If, according to the results, the article turned out to be unique, and the spelling meets the requirements of the Russian language, the publication is valid. The verification service account itself is referred to as a validator.

Manufacturing Process Validation
Thinking about the pharmaceutical industry or industry, it is easy to see that the word validation means that the product meets all the requirements of the manufacturer, so as not to shake his impeccable reputation in the market for services and goods. Simply put, the company is responsible for the high quality and correct manufacture of products that must meet the stated standards:
- For example, the release of a car takes place after checking all the components, in accordance with international requirements.
- The validator confirms the declared specifications, personal data, and the passed testing makes the car valid.
- The buyer, carrier or intermediary, in case of a mismatch in the parameters, can file a claim with the validator. Then additional testing of the goods in production is carried out.

What is the difference between verification and validation
In simple terms, both terms have a similar meaning, they are synonyms. Many simple examples can be given on this topic, but there is still a significant difference. If validation is a comprehensive check of the goods, then verification focuses more on the observance of the technological process, the consistent implementation of all stages of production. When the finished product does not satisfy a person in terms of quality parameters, the word verification does not matter to the stated claim.
The complexities of interpreting ISO 9001's requirements for "special process validation" are known to every quality manager who has ever encountered the implementation of this standard. So it turns out in practice that it is quite a standard requirement, clearly and in detail described in normative documents for the pharmaceutical industry, for other industries is accompanied by a frightening amount of interpretation and clarification. Extract from ISO 9001 clause 7.5.2: “An organization should validate any process for producing a product or providing a service whose final output cannot be confirmed (verified) by subsequent monitoring or measurement, and therefore whose deficiencies (i.e. the final output) are revealed only after the start of use of the product or the completion of the provision of the service ». And accordingly, in the ISO 9000:2005 standard, in the 3rd note to the definition of the term “process” (3.4.1), it is stated that “The process in which confirmation (verification) of the conformity of the final product cannot be carried out in a timely manner or entails significant economic costs is often called special”.
For the pharmaceutical industry, the definition of a special process fully falls under the "technological process", i.e. drug manufacturing process. Naturally, it is assumed that the drug is of high quality. What is drug quality? First of all, it's his efficiency, safety and specification conformity(quality standard). Compliance with the specification can be confirmed by quality control (in fact, verification), but the problem is that the control is selective. Those. control results apply to the entire series based on testing of samples that are not sold. And it is even more of a challenge to prove that the sample is representative. Further even worse. The safety and efficacy of a medicinal product is confirmed (or not confirmed) only in the process of its use - i.e. then, when something is changed, it is already impossible to fix it.
That is why one of key principles(G ood Manufacturing Practice,) is considered a technological process. Process validation is covered in a separate appendix, Annex 15, which was included in 1987. And what is very important, without the results of validation, the commercial release of a medicinal product is impossible, to put it more strictly, it is prohibited. supports the concept of GMP in shifting the focus from finished product quality control to process quality assurance. In addition, the procedures for organizing and conducting validation reflect the basic principles of GMP, namely: thoughtful planning, precise execution and detailed documentation. includes such important elements for GMP as a scientific approach based on quality risk assessment and change management.
What is validation?
The root "valid" means suitable. In Russian, there are several words with this root, for example, “disabled” - unsuitable, “validny” - suitable. In the pharmaceutical industry, the term "validation" is interpreted as follows: " The process of documented evidence that a reasonable degree of assurance has been achieved that
- Manufacturing process,
— Analytical methods,
- The equipment used
— Production systems,
comply with the current GMP principles and fulfill their functional purpose, i.e. using them actually gives the expected results».
In fact, the validation of the technological process is the ultimate goal, to achieve which it is necessary to sequentially validate a number of other related processes. In GMP, the general term "validation" is divided into two concepts: "process validation" and "qualification of production systems". Qualification of production systems is a part of process validation aimed at documenting the suitability of equipment, engineering systems, and a set of premises that are used in the manufacture of a medicinal product. Qualification is carried out in order to be sure that production system does not affect the quality of the product, and also for the fact that if we get a negative result during the direct validation of the technological process, this cannot be due to equipment/system failures, and the reasons must be sought in the technological process itself). Logically, the qualification of production systems is a kind of preventive measure.

Thus, process validation in the pharmaceutical industry means:
— Qualification of cleanrooms
– Qualification of engineering systems (preparation of clean air, purified water and water for injection, compressed air, etc.)
— Qualification production equipment
– Qualification of analytical equipment (used for quality control of raw materials, semi-finished products and finished products)
— Qualification of storage areas (raw materials, finished products)
— Validation of computerized systems, including IT infrastructure qualification
— Validation of analytical methods
— Validation of cleaning of premises, equipment
— Validation of aseptic conditions
— Validation of the stages of the technological process
— Packaging validation
Organization of validation works
Responsibility for conducting validation work is usually assigned to the Quality Assurance Department. To coordinate activities structural divisions a Validation Commission and validation groups are being created.
Documentary support
Standard package of validation documentation:
— Validation Master Plan (or General Validation Plan)
— Validation dossier (separately for each object): User requirements specification (URS, User R equirement S pecification); Risk assessment protocol; Program (scenario) of validation works; Protocol/Report of validation work; Program of (scheduled, unscheduled) revalidation (re-validation)
Qualification
For each critical infrastructure item, qualification must be carried out, which, as a rule, is carried out in four successive stages:
(DQ) project suitability confirmation(design, design solution) of technical means, engineering systems and equipment for their intended use. Scope of work at this stage:
– Description of the system (function, equipment parameters, special characteristics)
- Technical documentation ( regulatory requirements, hardware documentation)
– Design assessment (structural materials, contamination risk assessment)
— Components/elements of equipment/systems
— Analysis of possible failures/defects
– Analysis of the manufacturing method (critical parameters of work in the manufacture of equipment, calibration requirements)
(IQ) focused on documented the confirmation Togo,
what technical means, engineering systems and equipment are designed, equipped and installed in accordance with the working documentation of the project and the manufacturer's recommendations. Scope of work at this stage:
— Availability of sufficient documentation
— Availability of all items in the delivery
- Correct installation and connections
— Compliance of contact materials
— Compliance of measuring instruments
(OQ) focused on documented the confirmation the fact that technical means, engineering systems and equipment function properly over the entire declared range operating characteristics. Scope of work at this stage:
— Acceptability of documentation (operating, maintenance instructions);
— Tests involving a condition or set of conditions covering upper and lower limits of operating parameters:
- Operation of blocking / alarms.
As a rule, after this qualification stage, the object is put into operation.
Functioning qualification () carried out for engineering systems that operate continuously, as well as for equipment with complex control. Operational qualification is a documented the confirmation the fact that technical means, engineering systems and equipment, when used together (or for a long time), can reliably function with obtaining reproducible product properties.
At the same time, if the production system is equipped with an automated system for monitoring parameters, or data processing, an additional computerized system validation.
Validation of analytical methods
Each analytical and microbiological method that is used to control the quality of raw materials, semi-finished products or finished products must be validated. This means that we must obtain evidence of the suitability of such a technique for the control of a particular product and, accordingly, guarantee that reliable results will be obtained. In this regard, the GMP requirements are fully consistent with the requirements of ISO 17025.
Cleaning Validation
Equipment cleaning procedures must also be validated before we start manufacturing a drug on that equipment. First of all, this validation is aimed at obtaining assurance that high-quality cleaning can be carried out after the manufacture of such a product. In essence, this is minimizing the risk of cross-contamination when switching to the production of another product on the same equipment. If residues of the previous product remain on the equipment, this will not be detected - as there is no analytical control for the presence of such impurities.
Validation of aseptic conditions
in the production of sterile medicines using aseptic technologies, before the start of the technological process itself, it is necessary to confirm that throughout the entire process of manufacturing the drug (i.e. the duration of the process), not a single microorganism enters the product. Validation of aseptic conditions is carried out according to the simulation scenario using nutrient media.
Process Validation
And directly, the validation of each of the stages of the technological process is carried out on 3 consecutive series, taking into account the "worst case". And, very importantly, the validation of the technological process is carried out separately for each product and its claimed batch size. The worst case is carrying out the process under such conditions and circumstances (for process parameters, equipment operating modes) that have maximum chances of causing process rejection or product nonconformity compared to ideal conditions. The logic is very simple - if under such conditions we get a quality product, then we are guaranteed to achieve quality within the specified ranges.
Revalidation/qualification
After specified periods of operation (use), each object / process must be re-validated. The main purpose of revalidation (revalidation) is to obtain confirmation that the object/process continues to be in a valid state. This fully reflects the logic of GMP: “To confirm the quality of a product, it is not enough to carry out validation at the beginning of its life cycle needs to be monitored and continuously improved” (see chart below).

Scheduled and unscheduled revalidation is considered. Planned - carried out according to the schedule in accordance with a predetermined frequency (usually after 12-24 months). Unscheduled revalidation - after a long downtime, when there is a trend of deviations or when changes are made.
The list is given in the sequence in which validation work should be carried out.
13th international project"Constellation of Quality'2012"
Alexander V. Alexandrov, VIALEK Group of Companies
Abstracts of the report
The concepts that we will thoroughly analyze are quite often found both in everyday life and in specialized literature, professional activity. Many people want to know, verification and validation - what is it in simple words? What is the difference between these terms? Let's reason together.
Validation and verification - what is it in simple words?
Both concepts are related to testing a product and ensuring its quality. If we speak in simple terms, we will deduce the following:
- Validation is a guaranteed confidence of the manufacturer that he created the product according to all the necessary standards.
- Verification - helps to make sure that the product meets all the initially specified requirements for it.
Telling in simple words that this is verification and validation, you need to focus on the following facts:
- For the consumer, the most important thing is validation - the assurance that he receives the right product that meets his requirements.
- For the manufacturer, verification will be more valuable - confirmation that the product that he sends for sale meets all the necessary standards and norms.
Another meaning
We will analyze the difference in the concepts of "verification" and "validation" in testing. Indeed, by and large they are associated with international requirements for verification, acceptance of technologies and various products.
However, at the same time, words have firmly entered the life of Internet users. For example, when registering in payment systems such as Qiwi, Yandex.Money, you must go through the verification process. In this case, this means authenticating the specified data about yourself, identifying you by the system.

And those who actively use social networks("VKontakte", "Odnoklassniki", etc.), sooner or later they see a window in front of them asking them to pass the validation. This is the same verification of the truth of the data you entered. For example, the phone linked to the account receives an SMS with a code that you need to type in a specific field to confirm that you are the owner of the specified number.
Thus, in this case it is difficult to distinguish the difference between validation and verification. Both, in fact, here is a check for an indication of the data corresponding to reality. We would also like to point out the fact that developers of various viruses successfully use validation/verification in order to extort personal information from you. Why such data should be entered on reliable resources, from a computer protected by modern high-quality antivirus.
Definition of ISO 9000:2000
To explain in simple terms that this is verification and validation, the characteristics of these terms given in ISO documents (ISO - International Organization for Standardization) will help. Here we see the following:
- Verification is confirmation, on the basis of objective facts provided, that the established norms have been met.
- Validation is confirmation, on the basis of objective facts provided, that the established standards for a particular application are met.

From these definitions, the difference between validation and verification already follows:
- The first procedure is carried out only when necessary. The product is analyzed under specified operating conditions. The result will be a verdict: is it possible to use it in this environment.
- The second procedure is almost mandatory. This is a test for product compliance with requirements that will be relevant under any conditions, for any use.
Other definitions of verification
To help us understand the topic, a number of common definitions of the concepts under consideration will help us. Here are the characteristics of verification:
- Confirmation of conformity of the released goods, product to certain standards.
- Practically obligatory procedure; comparison of the characteristics of the produced unit with a set of specified requirements. The result is a verdict of compliance or non-compliance with the latter.
- Proclamation of confirmation that the established norms regarding the product have been met.
- In simple words, a product has been created that meets the required standards.

Other definitions of validation
Consider now the definitions of validation:
- The practical determination of how a particular product meets the expectations of its direct users.
- procedure to be carried out as needed. This is a common analysis of given conditions and an assessment of the performance of a product in relation to its operation in a given environment. The result is a conclusion about the possibility of using the product, invention in a certain area.
- Confirmation of compliance with the requirements of the system of standards, the customer, the direct user, etc.
- In simple words, the right product has been created that satisfies the consumer.
Translation based differences
To determine the difference between validation and verification, reference to the translation of these words, which have English roots, will also help:

- Verification - any verification.
- Validation - giving something legal force.
Even from this it follows that verification precedes validation, is not final. It is the latter that gives the final verdict to the product, which has legal force.
Differences between verification and validation in comparison
AT comparative table it is easier to identify the differences between these somewhat similar terms.
| Verification | Validation |
| Are we making products right? | Have we produced the right product? |
| Has all functionality been implemented? | Has the functionality been implemented correctly? |
| Verification precedes validation: it includes a full check of the correctness of writing, production and other creation. | It happens after verification - the quality of the manufactured product. |
| Conducted by developers. | Conducted by testers. |
| Statistical type of analysis: comparison with established product requirements. | Dynamic type of analysis: the product is tested in operation to determine its compliance with the norms. |
| Objective assessment: based on compliance with certain standards. | Subjective assessment: a personal assessment given by a tester. |
Let's talk a little more about the difference between validation and verification in the next section.
Key differences between concepts
So, let's dot all the i's. Verification is any testing that a product goes through. Checking the correctness of the technology of its production, as well as the quality of the product. Validation is a concept closer to certification. This correspondence to some specific, and not general requirements. How good a product is not in general, but specifically for a particular consumer, customer or given conditions.
It can also be noted that verification is a paper, theoretical testing of a technology or product. Validation is a real, physical verification carried out in practice, in specific conditions.

If the product has passed verification, it means that it complies with some given technological requirements. If the validation is successfully passed, it turns out that in practice it is also applicable without any complaints. From this we can conclude that the latter concept is somewhat more important, more indicative than the first.
Verification examples
Let's look at specific examples to reinforce the difference between these concepts.
Pharmaceutical factory checks drugs for compliance with specific requirements. At the start of production, their safety for the patient in certain doses, the absence of a placebo effect, the lack of the possibility of destructive addiction, and so on, are established. Thus, the drug verification was passed. And the validation in this case is already carried out by the treating doctor: he determines whether the medicine will help a particular patient, whether its use will lead to a risk to the life and health of this person, etc.
Let's take a bicycle as an example. We check if there is a steering wheel, seat, chains, wheels, brake system, etc. Everything is in place? Verification passed!
Validation examples
Now examples of how validation differs from verification.

Any enterprise, in accordance with certain requirements, produces universal pipes. A question comes from the customer: is it possible to lay this product on the bottom of the sea? The manufacturer must validate his pipes according to the proposed conditions in order to objectively answer this question.
On the example of the same bicycle, it is also very easy to consider validation. Can the device be ridden? Can you slow down? Can you turn right or left? Switch speed? If everything is possible, the validation passed. We could not slow down, the seat fell, the steering wheel was loose - alas, the bike did not go through this procedure.
So we sorted out the concepts of "verification" and "validation", trying to express everything in simple language. We hope that this will help you clearly trace the difference between them, the features of each.
- (12.10) The manufacturer should document the overall validation policy, objectives and principles, including validation. technological processes, cleaning procedures, analytical procedures, in-process control procedures, computerized systems and in relation to those responsible for the development, verification, approval and documenting each step of the validation.
- (12.11) Critical parameters and/or characteristics should normally be determined at the design stage or based on prior experience; the ranges of values of these critical parameters and (or) characteristics necessary to ensure the reproducibility of the process should also be determined. In this case, it is necessary:
- determine the critical characteristics of the API as a product;
- indicate the process parameters that can affect the critical quality indicators of the API;
- set the range of values for each critical process parameter to be used in batch production and process control.
- (12.12) Operations that are considered critical to the quality and purity of an API should be validated.
Validation documentation (12.2)
- (12.20) For each process to be validated, a validation protocol should be developed. This protocol should be reviewed and approved by the quality unit(s) and other relevant units.
- (12.21) The validation protocol should define critical process steps and acceptance criteria, as well as the type of validation to be performed (eg retrospective, prospective, concurrent) and the number of production runs.
- (12.22) The validation report should cross-reference the validation protocol and summarize the findings, explain any deviations found, with appropriate conclusions, including recommended changes to correct the deficiencies.
- (12.23) Any deviations from the validation protocol should be documented with appropriate justification.
- (12.30) Prior to commencement of process validation work, the qualification of critical equipment and auxiliary systems. Qualification is usually carried out in the following stages (individually or in combination):
- design qualification: documented evidence that the proposed design of a plant, equipment, or system is fit for its intended use.
- installation qualification: documented confirmation that the installation of premises, systems and equipment (installed or modified) was carried out in accordance with the approved design, manufacturer's recommendations and (or) requirements of the drug manufacturer.
- performance qualification: documented evidence that facilities, systems and equipment (whether installed or modified) perform as intended under all intended modes of operation.
- Performance Qualification: Documented evidence that facilities, systems and equipment, when used together, operate efficiently and reproducibly in accordance with specified requirements and process characteristics.
Process Validation Approaches (12.4)
- a) (1) critical quality indicators and critical process parameters are defined;
- b) (2) appropriate acceptance criteria and in-process controls are established;
- c) (3) there have been no significant process failures or product defects due to causes other than operator error or equipment failure;
- d) (4) impurity profiles were established for this API.
- (12.45) Batches selected for retrospective validation should represent a representative sample of all batches produced during the period under review, including any batches that do not meet specifications. In this case, the number of such series should be sufficient to prove the constancy of the process. Archival samples may be tested to provide data for retrospective process validation.
Process validation program (12.5)
- (12.50) The number of production runs required for validation should depend on the complexity of the process or the significance of the process changes to be considered. For prospective and concurrent validation, data obtained from three consecutive production batches of products should be used. good quality. However, there may be situations when additional data are needed to prove the stability of the process. production cycles(e.g. complex API manufacturing processes or lengthy API manufacturing processes). To assess the consistency of the process in a retrospective validation, as a rule, it is necessary to examine data for 10 - 30 consecutive series, but with appropriate justification, this number can be reduced.
- (12.51) During process validation studies, critical process parameters should be monitored and verified. Process parameters that are not related to quality, such as variables controlled to reduce energy consumption or equipment usage, may be omitted from process validation.
- (12.52) Process validation should confirm that the impurity profile for each API is within specified limits. The impurity profile should be similar to (or better than) the previously obtained profile, and also (where applicable) the impurity profile established during the development of the process or batches used for basic clinical and toxicological studies.
Periodic review of validated systems (12.6)
- (12.60) Systems and processes should be subject to periodic evaluation to confirm that they continue to function correctly. If the process or system has not been introduced significant changes and the quality review confirmed that the system or process consistently produces material that meets specifications, usually without the need for re-validation.
- (12.70) Cleaning procedures should normally be validated. Purification validation is carried out in cases where contamination or transfer of substances poses the greatest risk to the quality of the API. For example, equipment cleaning procedures may not need to be validated early in the process if residues are removed in subsequent cleaning steps.
- (12.71) Validation of cleaning procedures should reflect the actual use of the equipment. If different APIs or different intermediates are produced in the same equipment and the equipment is purified in the same way, then representative intermediates or APIs can be selected for purification validation. Such a choice should be based on solubility data and difficulty in purification, as well as on the calculation of the residue limits, taking into account their activity, toxicity and stability.
- (12.72) The cleaning validation protocol should describe the equipment to be cleaned, procedures, materials, acceptable cleaning levels, controlled and controlled parameters, and analytical methods. The protocol must also indicate the types of samples taken, the methods of their selection and labeling.
- (12.73) For the detection of both insoluble and soluble residues, sampling methods should include, as appropriate, swabs, swabs, or other methods (eg direct extraction). The sampling methods used should be capable of quantifying residue levels on equipment surfaces after cleaning. The swab sampling method may not be practical if product contact surfaces are difficult to access due to design features equipment (e.g. interior surfaces of hoses, transport piping, narrow hatch reactor vessels, and small complex equipment such as micronizers and micronebulizers) and/or where there are process limitations (e.g. handling toxic substances).
- (12.74) Validated analytical methods should be used that are sensitive enough to detect residues or contaminants. The detection limit of each analytical procedure should be sufficient to detect a certain acceptable level of residue or contaminant. For the method, it is necessary to establish the achieved level of extraction of the substance. Residue limits should be realistic, achievable, verifiable and based on the content of the most harmful residue. Limits can be set based on the minimum amount of known pharmacological, toxicological or physiological activity of the API or its most harmful component.
- (12.75) For processes where there is a need to reduce the total number of micro-organisms or endotoxins in the API, or for other processes where such contamination may be of concern (e.g. production of non-sterile APIs used in the manufacture of sterile medicinal products), purification studies and/or ) sanitization of equipment should be carried out in relation to contamination by microorganisms and endotoxins.
- (12.76) The manufacturer should monitor cleaning procedures at specified intervals after validation to ensure that these procedures are effective when used during the current manufacturing process. The cleanliness of the equipment, when practicable, should be monitored by analytical testing and visual inspection. Visual inspection can detect significant concentrations of contaminants in small areas that may not otherwise be detected by sampling and/or analysis.
Validation of analytical methods (12.8)
- (12.80) The analytical methods used should be validated. The suitability of all test methods used must, however, be verified under actual conditions of use and the results documented.
- (12.81) Method validation should be carried out taking into account the characteristics given in the validation guidelines for analytical methods. The extent of analytical validation performed should depend on the purpose of the analysis and the stage of the API manufacturing process.
- (12.82) Appropriate qualification of the analytical equipment should be carried out prior to the validation of analytical procedures.
539. (12.83) Complete records should be kept of any changes to a validated analytical method. Such records should reflect the reason for the change and relevant data to demonstrate that the change produces results that are as accurate and reliable as those obtained using the accepted methodology.
How to validate production processes and how important is it to distinguish “special” ones from them?
Kachalov V.A.
Journal "Methods of Quality Management", 2010, No. 10-11
Download (size: 380.3 Kb, downloads: 2724 )
This post is another attempt at an answer.
to the "tricky" questions of a meticulous quality manager
This article will focus on how to interpret the requirements of clause 7.5.2 of the ISO 9001:2008 standard.
The author analyzed 100 successive quality manuals of companies certified in TUV International Certification, as well as several companies certified in other certification systems, and found the following.
In 57% of cases, it was stated that the requirements of clause 7.5.2 do not apply, since the company does not have production and service processes, the results of which cannot be verified by subsequent monitoring or measurements 1.
These companies carried out mining and processing of ore and coal, production of metal, perfumery and cosmetic products, uranium enrichment, gas transportation, overhaul of wells, radioactive waste management, architectural design, production of glass and plastic products, provision of services for technical diagnostics, servicing of air passengers, production of building structures, food products, as well as dispatching and power supply, mechanical engineering, instrument making, production of rubber products, construction and installation works.
In the remaining 43% of cases, it was stated that the above processes were in place and that they had been validated in accordance with the requirements of clause 7.5.2. This included representatives of petrogeophysics, electrical engineering, automotive, pipe production, as well as dispatching and power supply, mechanical engineering, instrument making, production of rubber products and construction and installation works.
Thus, we can say with great confidence that the requirements of clause 7.5.2 of the ISO 9001:2008 standard are applied by 40 to 50% of certified companies, which means that in Russia we are talking about many thousands, if not tens of thousands of such companies.
However, the analysis shows the following:
First. Representatives of the same industry (they are highlighted in bold above), using almost the same technologies, nevertheless, differently assess the presence of processes that require the application of the requirements of clause 7.5.2.
Second. Many organizations apply “own” interpretation of the content and meaning of process validation activities, including those required by the standard demonstrating the ability of processes to achieveplanned results, for example:
The company has defined production processes as special processes, the results of which cannot be verified through consistent monitoring or measurement. THE CAPABILITY OF THESE PROCESSES TO ACHIEVE THE PLANNED OUTCOMES SHALL BE CONFIRMED THROUGH THE APPLICATION OF THE APPROPRIATE EQUIPMENT AND SKILLS OF PERSONNEL, THE APPLICATION OF SPECIFIC METHODS AND PROCEDURES AND THE KEEPING OF RELATED RECORDS.
Validation of the main processes... PROVIDED BY A LICENSE educational activities, attestation certificate educational institution, certificates of state accreditation 2 .
In the Quality Manual of one construction organization The section "Validation of production and service processes" contains 8 subsections, but quoting them does not make sense, since in fact none of them has anything to do with process validation activities.
Third. The explanations of the requirements of clause 7.5.2 of ISO 9001:2008 in the specialized literature are extremely brief and not always clear. Here, for example, is the FULL text of the comments to this section in:
The requirements of this subclause apply when it is not possible to measure parameters and monitor processes in accordance with subclause 8.2.3 "Monitoring and measurement of processes".
This subclause of the standard applies to situations where it is not possible to evaluate the output of the process or if such an assessment can only be obtained after a long time.
It is the organization's responsibility to assess in advance the likelihood of such situations occurring and to have appropriate controls in place for such processes.
No additional information on this question we will not find even in a large monograph. Commenting on the requirements under discussion, its author simply recounted the relevant requirements of the ISO 9001 standard in a very abbreviated version.
If we talk about targeted research, for example, about publication, then, as will be shown below, they contain many controversial statements.
Fourth. Requirements for the validation of production and service processes are not only contained in the ISO 9001:2008 standard. They are also included in the content:
Other international standards based on the requirements of ISO 9001, such as ISO/TS 16949:2009 and ISO/TS 29001:2003;
Agreements developed during international workshops (International Workshop Agreement - IWA), for example IWA 2:2003 and IWA 4:2005;
international standards published by other international associations, such as IRIS.
All these circumstances confirm the relevance of the search for answers to the questions posed in the title of the article.
1. How to validate production processes
1.1. Clause 7.5.2 requirements of ISO9001:2008 and "special processes"
Virtually all discussion of the requirements of clause 7.5.2 is usually related to the concept of special processes, although in its text there are no direct references to these processes. Moreover, in this section of ISO 9001:2008 and in ISO 9000:2005, where the concept of “special process” is given, they say, at first glance, not exactly the same thing:
ISO 9001:2008 refers to the processes the results of which CANNOT be verified by subsequent monitoring or measurements;
ISO 9000:2005 refers to the processes in which verification of conformity of the resulting product is DIFFICULT or ECONOMICALLY UNREASONABLE.
And "can't" “difficult or not economicallyexpedient" like different things.
In fact, both sources say, of course, about the same thing. First, because "difficult or not economically viable," including their extreme case of "impossible under present conditions," constitute an exhaustive list of reasons why "can't."
Secondly (and this is the main thing), both in the first and in the second case of processes, verification of their results is NOT PLANNED and IS NOT CARRIED OUT. And that's why deficiencies in a product or service cannot be identified during or after their creation, and become noticeable only after the product has been used or the service has been provided.
Conclusion one. The requirements of clause 7.5.2 apply only to those PRODUCTION PROCESSES that, in accordance with the terminology of the ISO standard9000:2005 are categorized as "special". Strictly speaking, the requirements of this section have nothing to do with any other processes.
The key feature of special processes is that when they are APPLIED, the results obtained, for one reason or another, ARE NOT subject to verification. This EXCLUDES IN PRINCIPLE the possibility of confirming or not confirming the conformity of these results with the requirements that refer to their particular presupposition my use or application, BEFORE they start use or application.
With this in mind, the importance of the requirement of ISO 9001:2008 that each special process be validated in advance, namely before its application, becomes clear.
1.2. Content and purpose of validation of production processes
Unfortunately, applying the term “validation” to processes as defined in ISO 9000:2005, namely: confirmation (by providing objective evidence) that the requirements relating to a specific intended use or application have been met,- causes quite certain difficulties. To help overcome this difficulty, publications attempt to define the essence of process validation 3 , including how to:
validation, approval, legalization, ratification [ 15];
procedure that gives a high degree confidence that a particular process, method or system will consistently produce results that meet predetermined acceptance criteria;
(from lat. valius - strong, healthy, corresponding to healthy) - documented action confirming that the methods, processes, activities or systems used in research or production actually lead to the expected results in accordance with the regulatory documentation;
obtaining documented evidence that gives a high degree of confidence that the process will consistently produce a product that meets predetermined requirements and quality criteria;
proof that something (process, etc.) is working as it should. In other words, the process must work in accordance with its purpose. To prove that this is the case, a system of sequentially performed checks and tests, called validation, is used..
The above definitions help to understand that to validate a process means to carry out control actions to find out whether the process developed “on paper” has the following inherent property: when applied in strict accordance with the developed technology, it allows obtaining results that meet the requirements for their specific intended use or application. If the specified evidence of compliance is obtained, the process is recognized as validated.
Second conclusion. The CONTENT of a process validation activity is the activity of evaluating the conformity of its outputs to the requirements for their particular intended use or application, and the PURPOSE of validation is to obtain confidence that the process has a sustained intrinsic capability to achieve this conformity when applied in the future.
1.3. Validation of production processes and product validation
Process validation requires actions to assess the compliance of its results (manufactured products or semi-finished products) with certain requirements. And this, in the language of the ISO 9000:2005 standard, is verification activities. At the same time, it is easy to notice: if during the VERIFICATION of manufactured products into the composition established requirements will be included requirements relating to a specific intended use or application, then such verification automatically becomes a product VALIDATION.
This means that: when the acceptance criteria for products produced by a process (including intermediate ones) include requirements, relating to its specific intended use or application, and upon completion of the process, the manufactured product MAY and SHOULD be verified against such acceptance criteria, then the positive results of this verification will simultaneously be evidence of validation of both the product itself and its production process. Moreover, it is on this principle that the commonly used mechanism for recognition, approval, etc. (ultimately, validation) of production processes is built - on the validation of products produced with their help 4 .
Another thing is if the received products for one reason or another CANNOT be subjected to the verification procedure. How to be in this case? This is indeed a question, moreover, the most important one for this part of the article. This is what will be discussed below.
Conclusion the third. The positive results of product validation are direct evidence of the validation (principal acceptability for its application) of its production process, because thereby they demonstrate that this process has an internal ability to receive what was required of it.
1.4. Validation of a specific process and authorization for its specific application
A special process, like any other, is developed for its subsequent APPLICATION. However, it is hardly advisable to start its implementation if it does not have TWO consecutively obtained permits for use - basic and private. This is what the content of clause 7.5.2 points to.
1.4.1. Basic permission to use
It is required by the first paragraph of this section of the standard, which refers to the need for validation of special processes. Let us explain the meaning of this statement.
Validation, as mentioned above, confirms that the process has an INTERNAL CAPACITY to achieve the desired results. However, this confirmation must be based on demonstrating the ability of the process to achieve planned results, based on established criteria. Therefore, the author shares the point of view of those who claim that process validation is the basis for its OFFICIAL approval.
At the same time, it is extremely important to understand WHAT EXACTLY happens during the process validation of the production process.
The acquisition of this status is possible only when, during the implementation of this process:
a) PERFORMERS of work, whose competence was not lower than the requirements established in the description of the developed process,
b) using EQUIPMENT having characteristics no worse than those established in the description of the developed process,
c) from RAW MATERIALS, MATERIALS, COMPONENTS, having characteristics no worse than those established in the description of the developed process,
d) in a WORKING ENVIRONMENT that is no worse than those specified in the design process description, and
e) in strict accordance with the TECHNOLOGICAL CHARACTERISTICS OF OPERATIONS (temperature, current, chemical composition etc.), which are established in the description of the developed process PRODUCTS WAS OBTAINED (including intermediate ones) that meet the requirements that relate to its specific intended use or application. In other words, at least once in SUCH conditions of its application, the process really gave "output" what was wanted from it.
The application of “validated”, “authorized”, “attested” or “approved” to the process means that, AS submitted by the developers, it has at least once passed the validation procedure with positive results, on this basis it is officially recognized as acceptable and approved for future use. Validation signals that a process has basic (initial, fundamental) permission to use or permission to use the process as such 5 . It is PRINCIPALLY ready to use.
But, we will notice especially, AND ONLY.
1.4.2. Private permission to use
By itself, a specific “launch into production” of a special process, even a validated one, does not mean in any way that we will get what we wanted at the end of it.
If needed with big share confidence to get from the application of the validated process the same results that it is potentially capable of and which it “gave out” during its validation, it is necessary in EVERY case to ensure the existence of such conditions for its implementation that would be NO WORSE than those that were during its validation. Namely: to ensure that the competence of the participants in the work, the equipment used, raw materials, materials, components, the production environment and the technological parameters of the operations carried out meet the requirements established in the documentation of the validated process. In quality management theory, these components are known as the five "M" (Man, Mechanism or Machine, Material, Milieu, Method).
Receipt private license to use and means that the validated process has all the necessary "M" and is thus PREPARED to start its specific (separately taken, private) implementation with SUFFICIENT confidence in a positive outcome.
This is precisely what is stated in the third paragraph of clause 7.5.2, which requires the organization:
approved (recognized the acceptability) of the equipment, which they plan to use in this process, and personnel qualification, who will be involved in the implementation of this process;
applied specific (designed specifically for this process) methods and procedures, i.e. exactly those that have been validated and have a basic authorization to use;
Established records confirming the availability of both initial (validated technology, acceptable equipment, personnel, raw materials, materials and components) and supporting components of the process (compliance with technology and requirements for the production environment 6).
It is important to emphasize that private license to use is an INDEPENDENT approval required for a SPECIFIC application of the process. Not only does it not cancel, but it ASSUMES the existence of a validated process as a whole. At the same time, the analysis of the Quality Guidelines showed that the difference between basic and private permission for use is recognized and taken into account by far not everyone.
For example, one of the Quality Manuals states: THE ABILITY of these processes to achieve the planned results is confirmed THROUGH THE APPLICATION OF THE APPROPRIATE EQUIPMENTand staff qualifications, application of specific methods and procedures and maintaining relevant records.
Another says that VALIDATION of special processes INCLUDES:
certification of the equipment used;
study of the materials used;
training and certification of personnel.
In the third that CRITERIA FOR APPROVAL[in the sense of validation - approx. ed. ] process ARE:
results of personnel attestation;
equipment certification results;
fulfillment of the technological discipline control schedule and its results.
As you can see, in these cases, speaking about the actions to carry out the validation of the process, i.e., to obtain basic permission to use, organizations actually invest in them the meaning of actions to obtain
Conclusion four.The expression “validated process”, of course, means that this process is “approved”, “attested”, “authorized / allowed for use”, etc., since it has demonstrated at least once compliance with the VALIDATION CRITERIA - their ability to achieve planned results. But this is only a statement of the process “in principle”, meaning that it has a “BASIC permission to use”.
In order to succeed in a SPECIFIC application of a validated process, it is also necessary to ensure that there is also a “PRIVATE application authorization”. The basis for obtaining it will be the compliance of the process with OTHER CRITERIA, namely: providing confidence that this process, in this particular application, will be carried out under the same conditions as when it was validated.
In order to guarantee the fulfillment of any particular order, the special processes used must have BOTH of the specified application approvals.
1.5. Errors in recognizing special processes as validated
The analysis of Quality Manuals and publications showed that in many cases the validation of special production processes required in clause 7.5.2, i.e. obtaining basic permission to use, voluntarily or involuntarily, but is SUBSTITUTED or even REPLACED by receiving private license to use. A typical example of this is the process validation methodology of one of the organizations described in the publication.
It reasonably states that criteria/requirements are needed for the validation/validation of a specific process, and that 10 days before the date of validation, protocols are drawn up to confirm the compliance of factors with the established requirements, on the basis of which the commission makes a decision on the approval of a special process or on the need for its re-validation.
And all would be fine, but the evaluated factors here were not chosen product characteristics (but the organization provides communication services), and not the degree of compliance with their requirements related to a specific intended use or application. The analyzed factors were: equipment, personnel, techniques, software, work environment and resources. And checked they are in accordance with what has been established IN THE PROCEDURES for the provision of communication services. And this means that ACTUALLY actions were taken to obtain not a basic, but a private permit for use.
Therefore, if you strictly follow the methodology described in the methodology, then the decision taken in this organization nie on approval of the special process(i.e. process validation decision) actually has nothing to do with VALIDATION, since it is based solely on assessing the compliance of certain “M” with the requirements established in the process description, and not on assessing the compliance of the RESULTS of the process with the requirements for these results. But whether the relevant PROCEDURES (i.e. CONTENT) of the process were validated and in what way - these questions remained unanswered in this publication.
Fifth conclusion. It is not uncommon for organizations to validate a process called special, requiring evidence of compliance with its results. requirements relating to a specific intended use or application, are replaced by actions to obtain evidence of the fulfillment of the requirements ESTABLISHED by the developers of this process for performers, raw materials, equipment, production environment, etc., often called "approval" or "certification" of a special process. At the same time, the acceptability (validation) of the process itself is not analyzed, by default it is considered proven sometime in the past, is not supported by anything, and confirmation of this validation in the future (i.e., revalidation) is not planned in any way.
It is important to understand that during certification, such an approach should be assessed by auditors as non-compliance with the requirements of the first paragraph and the last subparagraph of the third paragraph of clause 7.5.2 of the ISO 9001:2008 standard.
In fairness, it should be noted that in some organizations, when defining the concepts of “validation”, “approval” or “attestation” of special processes, they take into account the need for BOTH of the circumstances discussed above, which is, of course, correct. It is also necessary to add here those specialists who, in their publications, defining the concept of “process validation”, also IMMEDIATELY combine the basic and private permission to use into “one package”, speaking of validation, for example, as documented confirmation fromcompliance of equipment, production conditions, technological process, quality of semi-finished product and finished product with current regulations and/or requirements of regulatory documentation. Unfortunately, however, selective analysis showed that such organizations and publications are the exception rather than the rule.
1.6. How to validate special processes?
In the language of ISO 9001:2008, validation means that an organization must in SOMETHING demonstrate the ability of special processes to achieve planned results. How this can be done contains the desired answer.
Unfortunately, in most of the analyzed "Guidelines ..." the most common option was when the text, without any additional comments or references to any procedures, simply stated that process validationProduct performance demonstrates the ability of processes to achieve planned results. And that's it! The question of HOW EXACTLY this ability was demonstrated remained unanswered there. But this is the most important thing.
It seems that the above features of special processes allow us to talk about two methods for their validation.
1.6.1. Validation based on product evaluation
Recall that special processes include, INCLUDING, those for which verification conformity of the resulting products is DIFFICULT. However, we note that this does not mean that it is IN PRINCIPLE IMPOSSIBLE. Moreover, due to the fact that such verification for a number of special processes is in principle technically possible, it is this technique that is used in most cases for their validation.
Here is a representative example from the Quality Manual describing how a special process is validated: through certain technical documentationAt intervals of time, product characteristics are subjected to instrumental control in order to validate the process and verify the product of this process.
At the same time, the attentive reader will say that this method of validation of production processes has already been described above and even named there as commonly used. Therefore, the question is whether there are any differences in the application this method in relation to special processes?
The answer is that there are no methodological differences between using this validation method for conventional and special manufacturing processes. However, there are FEATURES. They lie in the fact that it is usually (as it happens) to organize and verify the results of special processes is much HARDER than the results of other processes.
For example, in principle, it is POSSIBLE to check the results of the protective electroplating process on products intended for operation in extreme conditions. But for this it will be necessary “only” to place several samples in the appropriate conditions (directly or create them artificially), and after a certain period to apply destructive control methods that require special equipment and software, trained personnel, etc. Agree, this is much more difficult to organize and do than, for example, to analyze the conformity of the geometric characteristics of products after they have been machining using standard means of linear-angular measurements.
And yet, the main thing here is different: although rarely, organizations IN PRINCIPLE can afford to do this. And they do.
Thus, in one of the Quality Manuals it is stated that the validation of processes is based on the results of acceptance control and testing of an experimental batch of products manufactured:
a) in the absence of comments on the condition and operation of the equipment involved in the certified production process;
b) in the absence of control and measuring devices involved in the certified production process that have not passed verification (calibration) or with an overdue verification (calibration) date;
c) technological personnel who have the necessary qualifications;
d) under conditions when all technological parameters of production meet the requirements established in the certified process description.
In this company, the process is considered validated if the products manufactured under the specified conditions fully complies with the requirements established in the normative and technical documentation.
But as for the re-validation (re-validation) of special processes, which is mentioned in clause 7.5.2, then, taking into account the indicated problems, the method under consideration is unlikely to allow this to be done often. In any case, it is clear that checking the conformity to geometric dimensions can be afforded to be done much more often than checking the characteristics of the protective coating. There is no doubt that the developers of the ISO 9001:2008 standard had this circumstance in mind, allowing the organization itself to establish either the frequency or specific dates for the next actions for re-validation (re-validation) of special processes.
In the general case, the criteria for choosing the interval or time for the next scheduled validation of a special process is a reasonable balance between the importance of maintaining its status as validated and the ability to allocate appropriate labor, material, financial and other resources for re-validation. With this in mind, one of the companies found that the revalidation of special processes is carried out after a year, as well as within the first two months after the overhaul equipment involved in the implementation of this process. In another company, re-validation is carried out only after five years.
Conclusion six. Validation of special production processes based on the verification of the received products is methodically no different from the validation of other processes. However, the ability to allocate resources for validation and then re-validation of special processes is usually a significant problem, since verification of the results of special processes is usually more complex and time-consuming than the results of conventional production processes.
1.6.2. Validation based on predictive estimates
Well, if verification of the results of a special process is possible in principle, even if this causes additional difficulties. But there may be cases when this is IMPOSSIBLE - for technical reasons, due to time constraints or for economic reasons.
Examples related to technical and temporal circumstances can be the processes of creating a nuclear reactor vessel, which must withstand a colossal radiation load for 30 years without losing its strength characteristics. To verify these characteristics in full, it is necessary to load a real reactor into the created vessel, ensure that it operates for 30 years, and only after that measure the strength characteristics of interest. Obviously, this is simply impossible - neither technically nor in terms of the amount of time required.
An example of economic constraints would be the case when, in order to validate a process, it is necessary to actually destroy the created product, and it is very expensive and ordered in only one or a few copies. It is clear that under these conditions, the manufacture of another product EXCLUSIVELY for the validation of its production process, which will be destroyed in this case, is hardly economically justified.
In such cases, it is more reasonable to discuss and consider only the "hypothetical" result of a special process. At the same time, here, too, with a certain error, it is possible to analyze the acceptability of various predictive variants of these results obtained on the basis of a number of well-known methods: generalization of expert points of view, analogies, computational and experimental extrapolation, modeling of the course of the processes themselves and conditions for subsequent application or use received products, etc.
Of course, here the uncertainty of positive conclusions about the validation is much higher than in the direct verification of "live" results of the process. Therefore, with the emergence of any new scientific and technical information and / or new experience related to the methods of validation based on predictions, it is necessary to immediately analyze the adequacy of the previously applied methods and, depending on the results of the analysis, decide on a re-validation, taking into account those that have become available. new knowledge.
Seventh conclusion. In the absence of the possibility of validating special processes based on direct verification of products created on their basis, their validation should be carried out using predictive methods..
Because of the apparently higher uncertainty in the resulting estimates, an organization should consider revalidating special processes whenever new knowledge about the predictive methods used to validate those processes becomes known.
1.7. Are all processes called “special” really so?
The answer to the question of what a "special process" is is given above. However, not everyone shares this point of view.
For example, in the publication, special processes include processes, the results of which will be hidden during the execution of subsequent processes (stages, steps). However, even further, the authors, speaking about obtaining objective evidence necessary for the validation of SUCH processes, refer to evidence results of ACCEPTANCE and/or SURVEY of hidden works, separately presented operations, operational control. This means that verification of the results of these processes is not only possible, but also IS CARRIED OUT, moreover, in fact, ALWAYS. And this does not fall under the signs of special processes, described in the ISO 9000:2005 standard.
A large part of the disputes is related to the presence or absence of special processes in the provision of services. For example, the authors refer to special processes that include providingservices in real time and are performed directly at the point of contact with the consumer (for example, check-in and check-out of the client at the hotel or ensuring the repair of products at the location of the client).
Similarly, speaking of the process of providing educational services, the authors do not question the fact that educationalactivity is a process whose results cannot be verified by subsequent monitoring or measurement.
The author believes that special processes are INITIALLY part of only those services that are provided PERSONALLY. As for the services provided in PRIMARY, in most cases there are no special processes, as the author has already spoken about earlier in the publication. Therefore, the author shares the opinion expressed in publications that consider processes as such, related to control and testing (X-raygraphics, color flaw detection, ultrasonic control, pressure testing, etc.). And the authors repeat word for word, and also support the authors, stating that product test methods refer to central objects of validation.
And here the author is ready to argue, believing that only those control processes that are based on the destruction / damage of the product under study, or that cannot be reused for the same product due to extremely high speed her aging.
In all other cases, the control RESULTS MAY be verified either by repeating the same control operations, or by using OTHER control methods. And, therefore, they do not fall under the definition of "special process".
There are many other examples of identification of some processes as special by some authors and disagreement with them by others, including the examples given at the very beginning of the article.
Conclusion eight. The question of whether a particular process is special or not is very often controversial and there are directly opposite points of view.
At the same time, it seems that such disputes are insignificant, since in fact it DOES NOT MATTER whether this or that production process is classified as special or not, generally speaking.
2. How important is it to distinguish “special processes” from the production processes?
2.1. Validation of production processes: is it only "special"?
If we carefully analyze the requirements of other sections of the ISO 9001:2008 standard, in particular clause 7.1, we will see that the standard ACTUALLY implies that validated, i.e. received basic permission to use, there were not only special processes, but in fact ALL processes for the production of products. Let's analyze the following three standard situations.
a) If the organization uses TYPICAL production operations/processes, then they are validated by definition, since they cannot be recognized as typical without their appropriate validation.
b) If an organization uses processes BORROWED from other organizations to produce products, it is reasonable to assume that no borrowing can be considered by the organization for itself until it receives evidence of the validation of these processes.
c) The most difficult situation is when the production processes are CREATED in the organization itself - in accordance with subparagraph b) of paragraph 7.1. Note, however, that in this case the standard requires the organization to:
1) established product-related requirements, including acceptance criteria. And it would be completely illogical to think that these requirements WILL NOT include those that relate to its specific intended use or application - both in relation to the final product, and in relation to its intermediate states or semi-finished products;
developed the processes necessary to create products(including, of course, the processes of PRODUCTION of products, and not just the processes of design, procurement, control, etc.), as well as established the records necessary to provide evidence that the processes for creating products meet the requirements. And here it would be illogical to think that these last requirements will NOT include the requirement to ensure that the received products meet the above acceptance criteria.
Therefore, an organization will consider acceptable only a developed manufacturing process that will ensure that the resulting product meets the requirements for its specific intended use or application. This means that it MUST be properly validated.
In view of the foregoing, in any organization operating under the ISO 9001:2008 model, all product manufacturing processes SHOULD initially be classified as validated 8 . For this reason, the statement of the authors |7, p. 33] that criteriania of any organization's processes to VAPIABLE is ONLY that they cannot be verified by subsequent monitoring and measurement.
Conclusion ninth. Although not stated directly, in the standard modelISO 9001:2008 by its ESSENCE assumes that ALL applied production processes should be subjected to the validation procedure. And with regard to those production processes that the organization itself develops, this, in fact, is a direct requirement.
2.2. Does the section require 7.5.2 validation of other QMS processes?
Based on the logic of quality management, then validation is necessary not only for all production processes, but also for ANY process in general, which is named in clause 4.1 of ISO 9001:2008 necessary for QMS. Otherwise, there will be no guarantee that, during the implementation of an unvalidated process, its “output” will correspond to the desired result.
Other authors also point out this circumstance, noting: quality philosophy states that the most cost-effective way of doing business is to apply the philosophy of special processes to all processes of the organization. The author fully shares this point of view.
At the same time, we emphasize once again that in the ISO 9001:2008 standard, the requirement for the need to validate processes is directly present only in clause 7.5.2 and is related ONLY to product manufacturing processes. This means that, strictly speaking, the validation requirement does not apply to any other processes than specific product manufacturing processes. In particular, this requirement does not apply to the design and development processes, although the latter are often referred to when discussing the problems of interpreting the term “validation”.
Conclusion ten. Requirements of clause 7.5.2 of the standardISO 9001:2008 relates exclusively to special processes for the production of products and their service and does not apply to any other processes required for the QMS.
2.3. How important is it not to make a mistake in attributing production process to "special"?
It is clear that if some production process is UNREASONALLY classified as special, this will be a methodological error. But, from the point of view of the objectives of quality management, this is “a mistake in better side". Since, after this, the requirements of clause 7.5.2 of the ISO 9001:2008 standard will have to be extended to such a process, namely: it will have to be VALIDATED and implementation will begin only after obtaining private permission for use. And this is good for the organization, no doubt.
But it is a completely different matter when the process is inherently special, but it was not identified as such and therefore the requirements of clause 7.5.2 with all the ensuing consequences were not extended to it. However, it seems that fears about the catastrophic consequences of these consequences are “greatly exaggerated”.
From what has been said above, the reader should take away that ANY production process must actually be validated before its use (which is what happens in “normal” companies in practice), and then carried out under controlled conditions, the content of which is indicated in clause 7.5 .one. And this means that for these processes, if they meet the requirements of paragraphs. 7.1 and 7.5.1, all the requirements of clause 7.5.2 will be fulfilled AUTOMATICALLY, except for just one point - about revalidation.
Conclusion eleven.If the organization FULLY fulfills the requirements of paragraphs. 7.1 and 7.5.1 standardISO 9001:2008, then not classifying some processes as “special” does not automatically entail non-compliance with the requirements of clause 7.5.2. Standard ModelISO 9001:2008 ACTUALLY forces the organization to both validate such processes and apply all other requirements of clause 7.5.2 to them (with the possible exception of revalidation requirements), without even making direct reference to it.
This leads to a very important consequence for organizations. If, during certification, auditors conclude that a process is inherently ad hoc, but the organization has not given it that status, this MUST NOT automatically result in non-compliance with the requirements of 7.5.2. It is quite possible that ACTUALLY all the requirements of this section in this organization are met in the course of implementing the requirements of paragraphs. 7.1 and 7.5.1. Auditors are required to find out ADDITIONALLY so that their decision on compliance or non-compliance with the requirements of clause 7.5.2 is fully justified.
And one more question.
What if in the organization, when analyzing the production processes, it is revealed that it has already been using some processes for a long time, which (based on the ISO 9000:2005 standard) should have been identified as “special”, but which were previously NOT MANAGED in fully as required in clause 7.5.2 of ISO 9001:2008?
The answer here is unequivocal: the organization must START managing these processes in accordance with the requirements of clause 7.5.2, starting with their OFFICIAL validation / revalidation.
Conclusion twelve.In all cases, it is advisable for any certified organization to periodically (for example, once a year) analyze its production processes in order to clarify those that meet the definition of “special” and extend the requirements of clause 7.5.2 of the ISO standard to them. 9001:2008.
2.4. So is it necessary to single out “special” production processes?
Taking into account the real practice of application in the SM K of the requirements of paragraphs. 7.1 and 7.5.2 and relying on the utility argument, the author does not hesitate to answer this question in the affirmative. Moreover, he would consider it rational to recognize ALL production processes as "special". But with one caveat. When recognizing a process as “special” and/or “highly important”, the organization should then:
1. INSTALL methodology for its validation, ACHIEVE its validation and INSTALLtransshipment mode. Thus, it can indicate the presence of basic permission to use this process.
2. Before each specific use, take steps to obtain private permission for this use on the basis of ESTABLISHED CRITERIA analysis and recognitionacceptability of the process for use, which should include at least recognitionsuitability of the equipment planned for use and the personnel involved.
Provide oversight throughout the process to ensure that only intended EXACTLY FOR THIS PROCESSmethods and procedures and RECORDS WERE KEEPED confirming ensuring both the fulfillment of process acceptance criteria and compliance with established methods and procedures. Conclusion thirteenth. Circle of production processes,that an organization considers critical or particularly important may be outside the scope of processes that are ad hoc. Classifying a process as critical implies Special attention for its preparation and implementation. The best mechanism to implement this is to extend the requirements of clause 7.5.2 of the standard to such processes. ISO 9001:2008.
Conclusion
Ideological. The appearance in the text of the ISO 9001:2008 clause 7.5.2 emphasizes the importance that the International Organization for Standardization considers it necessary to pay in organizations to special processes due to the increased risk associated with them for quality management due to their specificity.
Despite the fact that the rules for managing production processes, formulated in paragraphs. 7.1 and 7.5.1 are essentially the same as in 7.5.2, the implementation of the requirements of the last section allows the organization to be much more confident in minimizing the risks associated with a possible misunderstanding of the management features of just such processes. Taking into account the “criticality” inherent in special processes, such an approach seems to be fully justified.
The increased attention required by the standard for special processes should logically be extended to all other critical processes.
Methodical. When passing certification for each applied special process, it is necessary to demonstrate the presence of a combination of two approvals for their use. The first (basic) is in the form of evidence of the validation of the process, i.e., evidence of its ability, in principle, to achieve the planned results.
The second (private) is in the form of confirmation of the readiness of the process for its specific application. This usually happens as a result of the development and implementation of plans. technical training corresponding production. The criterion for the presence of this permission is the fulfillment of the requirements established in the description of the process for all components included in the process, namely: for performers, equipment, source materials, production environment, etc.
Neither of these two permissions replaces the other. Both must be available.
Practical. If a special process has both of the above permissions for use, the degree of confidence in obtaining the desired result will depend in the future on only one thing - how accurately the technological regimes established by the developers are observed. To be sure of this, the organization should establish and maintain appropriate records.
It seems that it is in these three circumstances that the key meaning of the requirements of clause 7.5.2 of ISO 9001:2008 is contained, which the author recommends that any organization extend to other processes important to the QMS.
1 Here and below, quotations from documents are in bold italic type. The selection of words and phrases in capital (capital) letters is done by the author. Citation of individual Quality Manuals with reference to the companies that developed them is done only in individual cases.
2 The cited provision was taken from the Quality Manual of the university, which was previously posted on its website, but by the time the article was being prepared for publication, for some reason it was removed from there.
3 Although there are some rather strange exceptions. For example, which began to be published in a specialized magazine on quality management issues “ Terminological dictionary technical regulation system” does not contain the term “validation”.
4 It should be noted that in the post-Soviet space, the term “process certification” has remained in use. It is often used in lieu of the term "validation". The problem is that in some cases it really is the equivalent of validation, for example when the certification of production processes is defined in the Quality Manual as documentary confirmation that the process, proceeding within the established parameters, ensures the effective, efficient and reproducible production of PRODUCTS THAT SATISFY THE ESTABLISHED REQUIREMENTS and quality standards. But in other quality manuals, the content of the actions called “process certification” cannot be attributed to its validation. This is discussed in more detail in later sections of the article.
5 Naturally, we do not consider here the situation when, for one reason or another, OUTSIDE the logic of quality management, some person, by virtue of the authority given to him, allows the use of an unvalidated process. In such cases it is necessary to speak not of "permission", but of "order".
6 Strictly speaking, Clause 7.5.2 is silent on materials, raw materials and components, as well as on the working environment. However, this seems to be a purely "textual" flaw in this section, since these requirements are contained in other sections of the standard, ALSO applicable to special processes - paras. 7.4 and 6.4.
7 Strictly speaking, 7.5.2 refers ONLY to the PRODUCTION processes of the product, and not to the CONTROL processes of the product (more on this below). The author included a discussion of these processes in the article only from the point of view of an additional illustration of errors in classifying some of the processes as special as such.
8 In some standards for quality management systems, the discussed provision from the category of recommendations was transferred to the category of DIRECT requirements. For example, clause 7.5.2.1 of ISO/TS 16949:2009 states: the requirements of section 7.5.2 shall apply to all manufacturing and service processes







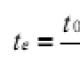


For beginners: breeding a broiler at home Boiled water for broilers
Only lovers will survive
Features of advertising aimed at children
retouching old photos in photoshop retouching old photos
What is an NPO: decoding, definition of goals, types of activities Does a non-profit organization have the right