To protect metals from corrosion, various methods are used, which can be conditionally divided into the following main areas: metal alloying; protective coatings (metal, non-metal); electrochemical protection; change in the properties of the corrosive medium; rational product design.
Alloying of metals. it effective method increase the corrosion resistance of metals. When alloying, alloying elements (chromium, nickel, molybdenum, etc.) are introduced into the composition of an alloy or metal, causing the passivity of the metal. Passivation called the process of transition of a metal or alloy to a state of its increased corrosion resistance, caused by inhibition of the anodic process. The passive state of the metal is explained by the formation of an oxide film with a perfect structure on its surface (the oxide film has protective properties under the condition of maximum similarity of the crystal lattices of the metal and the resulting oxide).
Alloying has found wide application for protection against gas corrosion. Alloying is performed on iron, aluminum, copper, magnesium, zinc, as well as alloys based on them. As a result, alloys with higher corrosion resistance than the metals themselves are obtained. These alloys have both heat resistance and heat resistance.
Heat resistance– resistance to gas corrosion at high temperatures. Heat resistance- the properties of the structural material to maintain high mechanical strength with a significant increase in temperature. Heat resistance is usually provided by alloying metals and alloys, such as steel with chromium, aluminum and silicon. These elements at high temperatures are oxidized more vigorously than iron, and thus form dense protective films of oxides, such as Al 2 O 3 and Cr 2 O 3 .
Alloying is also used to reduce the rate of electrochemical corrosion, especially hydrogen evolution corrosion. Corrosion-resistant alloys, for example, include stainless steels, in which chromium, nickel and other metals serve as alloying components.
Protective coatings. Layers artificially created on the surface metal products to protect them from corrosion are called protective coatings. The application of protective coatings is the most common method of combating corrosion. Protective coatings not only protect products from corrosion, but also impart a number of valuable physical and chemical properties to surfaces (wear resistance, electrical conductivity, etc.). They are divided into metallic and non-metallic. General requirements for all types of protective coatings are high adhesion, continuity and resistance in an aggressive environment.
Metallic coatings. Metal coatings occupy a special position, since their action has a dual character. As long as the integrity of the coating layer is not violated, its protective effect is reduced to isolating the surface of the protected metal from environment. This is no different from the action of any mechanical protective layer (painting, oxide film, etc.). Metallic coatings must be impervious to corrosive agents.
If the coating is damaged (or there are pores), a galvanic cell is formed. The nature of the corrosion failure of the base metal is determined by the electrochemical characteristics of both metals. Protective anti-corrosion coatings can be cathodic and anode. To cathodic coatings include coatings whose potentials in a given medium have a more positive value than the potential of the base metal. Anode Coatings have a more negative potential than the potential of the base metal.
So, for example, in relation to iron, the nickel coating is cathodic, and the zinc coating is anodic (Fig. 2.).
When the nickel coating is damaged (Fig. 2a), the process of iron oxidation occurs in the anode sections due to the appearance of microcorrosive galvanic cells. At the cathode sites - reduction of hydrogen. Consequently, cathodic coatings can protect the metal from corrosion only in the absence of pores and damage to the coating.
Local damage to the protective zinc layer leads to its further destruction, while the iron surface is protected from corrosion. Zinc oxidation occurs at the anode sites. At the cathode sections, hydrogen is reduced (Fig. 2b).
The electrode potentials of metals depend on the composition of the solutions; therefore, when the composition of the solution changes, the nature of the coating may also change.
Various methods are used to obtain metal protective coatings: electrochemical(electroplated coatings); immersion in molten metal(hot galvanizing, tinning); metallization(applying molten metal to the surface to be protected using a jet of compressed air); chemical(obtaining metal coatings using reducing agents, such as hydrazine).
Rice. Fig. 2. Corrosion of iron in an acid solution with cathode (a) and anode (b) coatings: 1 – base metal; 2 - coating; 3 – electrolyte solution.
Materials for metal protective coatings can be either pure metals (zinc, cadmium, aluminum, nickel, copper, chromium, silver, etc.) or their alloys (bronze, brass, etc.).
Non-metallic protective coatings. They can be either inorganic or organic. The protective effect of these coatings is reduced mainly to the isolation of the metal from the environment.
Inorganic enamels, metal oxides, compounds of chromium, phosphorus, etc. are used as inorganic coatings. Paint coatings, coatings with resins, plastics, polymer films, and rubber are organic.
Inorganic enamels they are silicates in their composition, i.e. silicon compounds. The main disadvantages of such coatings are brittleness and cracking under thermal and mechanical shocks.
Coatings the most common. The paintwork must be continuous, gas and watertight, chemically resistant, elastic, have high adhesion to the material, mechanical strength and hardness.
Chemical methods very varied. These include, for example, the treatment of a metal surface with substances that enter into a chemical reaction with it and form a film of a stable chemical compound on its surface, in the formation of which the protected metal itself takes part. These methods include oxidation, phosphating, sulfiding and etc.
Oxidation- the process of formation of oxide films on the surface of metal products.
The modern method of oxidation is the chemical and electrochemical treatment of parts in alkaline solutions.
For iron and its alloys, alkaline oxidation in a solution containing NaOH, NaNO 3 , NaNO 2 at a temperature of 135-140 ° C is most often used. Oxidation of ferrous metals is called bluing.
Fe  Fe 2+ + 2
Fe 2+ + 2 
At the cathode sites, the reduction process takes place:
2 H 2 O + O 2 + 4 
 4OH -
4OH -
On the metal surface, as a result of the operation of microgalvanic cells, Fe(OH) 2 is formed, which is then oxidized to Fe 3 O 4 . The oxide film on mild steel is deep black, and on high carbon steel it is black with a grayish tint.
Fe 2+ + 2OH -  Fe(OH) 2 ;
Fe(OH) 2 ;
12 Fe(OH) 2 + NaNO 3  4Fe 3 O 4 + NaOH + 10 H 2 O + NH 3
4Fe 3 O 4 + NaOH + 10 H 2 O + NH 3
The anticorrosive properties of the surface film of oxides are low, so the scope of this method is limited. The main purpose is a decorative finish. Blueing is used when it is necessary to maintain the original dimensions, since the oxide film is only 1.0 - 1.5 microns.
Phosphating- a method for obtaining phosphate films on products made of non-ferrous and ferrous metals. For phosphating, a metal product is immersed in solutions of phosphoric acid and its acidic salts (H 3 PO 4 + Mn (H 2 PO 4) 2) at a temperature of 96-98 o C.
As a result of the operation of microgalvanic cells, a phosphate film is formed on the metal surface, which has a complex chemical composition and contains sparingly soluble hydrates of two- and three-substituted manganese and iron phosphates: MnHPO 4, Mn 3 (PO 4) 2, FeHPO 4, Fe 3 (PO 4) 2 n H2O.
On the anode sites, the oxidation process occurs:
Fe  Fe 2+ + 2
Fe 2+ + 2 
At the cathode sites, the process of hydrogen reduction takes place:
2H + + 2 
 H 2
H 2  (pH< 7)
(pH< 7)
When Fe 2+ ions interact with orthophosphoric acid anions and its acid salts, phosphate films are formed:
Fe 2+ + H 2 PO - 4  FeHPO4+H+
FeHPO4+H+
3Fe 2+ + 2PO 4 3-  Fe 3 (PO 4) 2
Fe 3 (PO 4) 2
The resulting phosphate film is chemically bonded to the metal and consists of intergrown crystals separated by ultramicroscopic pores. Phosphate films have good adhesion and a developed rough surface. They are a good primer for applying paints and impregnating lubricants. Phosphate coatings are mainly used to protect metals from corrosion in enclosed spaces, and also as a method of surface preparation for subsequent painting or varnishing. The disadvantage of phosphate films is low strength and elasticity, high brittleness.
Anodizing- this is the process of formation of oxide films on the surface of the metal and, above all, aluminum. Under normal conditions, a thin oxide film of Al 2 O 3 or Al 2 O 3 ∙ nH 2 O oxides is present on the surface of aluminum, which cannot protect it from corrosion. Under the influence of the environment, aluminum is covered with a layer of corrosion products. The process of artificial formation of oxide films can be carried out by chemical and electrochemical methods. In the electrochemical oxidation of aluminum, the aluminum product plays the role of the anode of the cell. The electrolyte is a solution of sulfuric, orthophosphoric, chromic, boric or oxalic acids, the cathode can be a metal that does not interact with the electrolyte solution, such as stainless steel. Hydrogen is released at the cathode, aluminum oxide is formed at the anode. The overall process at the anode can be represented by the following equation:
2 Al + 3 H 2 O  Al 2 O 3 + 6 H + + 6
Al 2 O 3 + 6 H + + 6 
Electrochemical protection of metal structures from corrosion manifestations is based on the imposition of a negative potential on the protected product. It demonstrates a high level of efficiency in cases where metal structures are subjected to active electrochemical destruction.
1 The essence of anti-corrosion electrochemical protection
Any metal structure begins to break down over time as a result of corrosion. For this reason, metal surfaces are necessarily coated with special compounds consisting of various inorganic and organic elements before use. Such materials reliably protect the metal from oxidation (rust) for a certain period. But after a while they need to be updated (apply new compounds).
When the protective layer cannot be renewed, corrosion protection of pipelines, car body and other structures is carried out using an electrochemical technique. It is indispensable for rust protection of tanks and containers operating underground, the bottoms of sea ships, various underground utilities, when the potential for corrosion (it is called free) is in the zone of overpassivation of the base metal of the product or its active dissolution.
The essence of electrochemical protection lies in the fact that a constant electric current is connected to a metal structure from the outside, which forms a polarization of the cathode type of microgalvanic electrodes on the surface of the metal structure. As a result, the transformation of anodic regions into cathodic regions is observed on the metal surface. After such a transformation, the negative influence of the environment is perceived by the anode, and not by the material from which the protected product is made.

Electrochemical protection can be either cathodic or anodic. At the cathodic potential of the metal is shifted to negative side, at the anode - to the positive.
2 Cathodic electrical protection - how does it work?
The mechanism of the process, if you understand it, is quite simple. A metal immersed in an electrolytic solution is a system with a large number of electrons, which includes cathode and anode zones separated in space, electrically closed to each other. This state of affairs is due to the heterogeneous electrochemical structure of metal products (for example, underground pipelines). Corrosion manifestations are formed on the anode areas of the metal due to its ionization.

When a material with a high potential (negative) is attached to the base metal in the electrolyte, the formation of a common cathode is observed due to the process of polarization of the cathode and anode zones. In this case, a large potential is understood to be such a value that exceeds the potential of the anodic reaction. In the formed galvanic couple, the material with a low potential of the electrode dissolves, which leads to the suspension of corrosion (since the ions of the protected metal product cannot enter the solution).
Required to protect car body, underground tanks and pipelines, ship bottoms electricity can come from an external source, and not just from the functioning of the microgalvanic couple. In such a situation, the protected structure is connected to the "minus" of the electric current source. The anode, made of materials with a low degree of solubility, is connected to the "plus" of the system.

If the current is obtained only from galvanic couples, one speaks of a process with sacrificial anodes. And when using current from an external source, we are talking about the protection of pipelines, parts of vehicles and water vehicles using superimposed current. The use of any of these schemes provides high-quality protection of the object from general corrosion decay and from a number of its special options (selective, pitting, cracking, intergranular, contact types corrosion).
3 How does the anodic technique work?
This electrochemical technique for protecting metals from corrosion is used for structures made of:
- carbon steels;
- passivated dissimilar materials;
- highly alloyed and;
- titanium alloys.
The anode scheme assumes a shift in the potential of the protected steel in a positive direction. Moreover, this process continues until the system enters a stable passive state. Such corrosion protection is possible in environments that conduct electrical current well. The advantage of the anodic technique is that it significantly slows down the rate of oxidation of the protected surfaces.

In addition, such protection can be carried out by saturating the corrosive environment with special oxidizing components (nitrates, bichromates, and others). In this case, its mechanism is approximately identical to the traditional method of anodic polarization of metals. Oxidizing agents significantly increase the effect of the cathodic process on the steel surface, but they usually negatively affect the environment by releasing aggressive elements into it.
Anode protection is used less frequently than cathodic protection, since a lot of specific requirements are put forward for the protected object (for example, the impeccable quality of welded seams of pipelines or a car body, the constant presence of electrodes in solution, etc.). Cathodes in anode technology are arranged strictly certain scheme, which takes into account all the features of the metal structure.

For the anode technique, sparingly soluble elements are used (cathodes are made of them) - platinum, nickel, stainless high-alloy alloys, lead, tantalum. The installation itself for such corrosion protection consists of the following components:
- protected structure;
- current source;
- cathode;
- special reference electrode.
It is allowed to use anode protection for containers where mineral fertilizers, ammonia compounds, sulphuric acid, for cylindrical units and heat exchangers operated on chemical enterprises, for tanks in which chemical nickel plating is performed.
4 Features of tread protection of steel and metal
Quite often used version of cathodic protection is the technology of using special protector materials. With a similar technique, an electronegative metal is connected to the structure. During a given time period, corrosion affects the protector, and not the protected object. After the protector is destroyed to a certain level, a new "protector" is put in its place.
Protective electrochemical protection is recommended for processing objects located in soil, air, water (that is, in chemically neutral environments). At the same time, it will be effective only when there is some transitional resistance between the medium and the protective material (its value varies, but in any case it is small).

In practice, protectors are used when it is economically inexpedient or physically impossible to supply the required charge of electric current to an object made of steel or metal. It is worth noting separately the fact that protective materials are characterized by a certain radius to which their positive effect extends. For this reason, it is necessary to correctly calculate the distance to remove them from the metal structure.
Popular protectors:
- Magnesium. They are used in environments with a pH of 9.5–10.5 units (earth, fresh and low-salt water). Manufactured from magnesium-based alloys with additional alloying with aluminum (no more than 6–7%) and zinc (up to 5%). For the environment, such protectors that protect objects from corrosion are potentially unsafe due to the fact that they can cause cracking and hydrogen embrittlement of metal products.
- Zinc. These "protectors" are indispensable for structures operating in water with a high salt content. It makes no sense to use them in other media, since hydroxides and oxides appear on their surface in the form of a thick film. Zinc-based protectors contain minor (up to 0.5%) additions of iron, lead, cadmium, aluminum and some other chemical elements.
- Aluminum. They are used in sea running water and at facilities located on the coastal shelf. Aluminum protectors contain magnesium (about 5%) and zinc (about 8%), as well as very small amounts of thallium, cadmium, silicon, and indium.

In addition, iron protectors are sometimes used, which are made from iron without any additives or from ordinary carbon steels.
5 How is the cathode scheme performed?
Temperature fluctuations and ultraviolet rays cause serious damage to all external components and components of vehicles. Protection of the car body and some of its other elements from corrosion by electrochemical methods is recognized as a very effective way to extend the ideal appearance cars.
The principle of operation of such protection is no different from the scheme described above. When protecting the car body from rusting, the anode function can be performed by almost any surface that is capable of high-quality conduction of electric current (wet road surface, metal plates, steel structures). The cathode is directly the body of the vehicle.

Elementary methods of electrochemical protection of the car body:
- We connect through the mounting wire and an additional resistor to the plus of the battery the garage housing in which the car is standing. This protection from corrosion of the car body is especially productive in summer period when there is a greenhouse effect in the garage. This effect just protects the outer parts of the car from oxidation.
- We mount a special grounding metallized "tail" made of rubber in the rear of the vehicle so that drops of moisture fall on it while driving in rainy weather. At high humidity, a potential difference is formed between the highway and the car body, which protects the outer parts of the vehicle from oxidation.

Also, the protection of the car body is carried out with the help of protectors. They are mounted on the thresholds of the car, on the bottom, under the wings. Protectors in this case are small plates made of platinum, magnetite, carboxyl, graphite (anodes that do not break down over time), as well as aluminum and stainless steel (they should be changed every few years).
6 Nuances of anti-corrosion protection of pipelines
Pipe systems are currently protected by draining and cathodic electrochemical techniques. When protecting pipelines from corrosion according to the cathodic scheme, the following are used:
- External current sources. Their plus will be connected to the anode ground, and the minus to the pipe itself.
- Protective anodes using current from galvanic pairs.

The cathodic technique assumes the polarization of the protected steel surface. At the same time, underground pipelines are connected to the "minus" of the cathodic protection complex (in fact, it is a current source). "Plus" is connected to an additional external electrode using a special cable, which is made of conductive rubber or graphite. This scheme allows you to get a closed circuit, which includes the following components:
- electrode (outer);
- electrolyte in the soil where pipelines are laid;
- pipes directly;
- cable (cathode);
- current source;
- cable (anodic).
Materials based on aluminum, magnesium and zinc are used for the sacrificial protection of pipelines, coefficient useful action which is equal to 90% when using protectors based on aluminum and zinc and 50% for protectors made of magnesium alloys and pure magnesium.

For drainage protection of pipe systems, the technology of diverting stray currents into the ground is used. There are four options for drainage piping - polarized, earth, reinforced and straight. With direct and polarized drainage, jumpers are placed between the "minus" of stray currents and the pipe. For an earth protection circuit, it is necessary to make earthing by means of additional electrodes. And with enhanced drainage of pipe systems, a converter is added to the circuit, which is necessary to increase the magnitude of the drainage current.
The development of the steel industry is inextricably linked with the search for ways and means to prevent the destruction of metal products. Corrosion protection, development of new methods is continuous process in the technological chain of production of metal, products from it. Iron-containing products become unusable under the influence of various physical and chemical external factors environment. We see these effects in the form of hydrated iron residues, that is, rust.
Methods for protecting metals from corrosion are selected depending on the operating conditions of the products. Therefore stands out:
- Corrosion associated with atmospheric phenomena. This is a destructive process of oxygen or hydrogen depolarization of the metal. Which leads to the destruction of the crystal molecular lattice under the influence of a humid air environment and other aggressive factors and impurities (temperature, the presence of chemical impurities, etc.).
- Corrosion in water, primarily marine. In it, the process is faster due to the content of salts and microorganisms.
- The processes of destruction that occur in the soil. Soil corrosion is a rather complex form of metal damage. Much depends on the composition of the soil, humidity, heating and other factors. In addition, products, such as pipelines, are buried deep in the ground, which makes it difficult to diagnose. And corrosion often affects individual areas pointwise or in the form of ulcerative veins.
Types of corrosion protection are selected individually, based on the environment in which the protected metal product will be located.
Typical types of rust damage
Methods for protecting steel and alloys depend not only on the type of corrosion, but also on the type of destruction:
- Rust covers the surface of the product in a continuous layer or in separate areas.
- It appears in the form of spots and penetrates deep into the detail.
- Destroys the metal molecular lattice in the form of a deep crack.
- In a steel product consisting of alloys, one of the metals is destroyed.
- Deeper extensive rusting, when not only the surface is gradually broken, but penetration into the deeper layers of the structure occurs.
Damage types can be combined. Sometimes it is difficult to determine them immediately, especially when there is a point destruction of steel. Corrosion protection methods include special diagnostics to determine the extent of damage.
Allocate chemical corrosion without the occurrence of electric currents. In contact with petroleum products, alcohol solutions and other aggressive ingredients, a chemical reaction occurs, accompanied by gas emissions and high temperature.
Electrochemical corrosion is when a metal surface comes into contact with an electrolyte, in particular water from the environment. In this case, the diffusion of metals occurs. Under the influence of the electrolyte, an electric current arises, the substitution and movement of the electrons of the metals that enter the alloy occurs. The structure is destroyed, rust is formed.

Steel smelting and its corrosion protection are two sides of the same coin. Corrosion causes great harm to industrial and commercial buildings. In cases with large-scale technical structures, for example, bridges, power pylons, barrier structures, it can also provoke man-made disasters.
Corrosion of metal and methods of protection against it
How to protect metal? Corrosion of metals and ways to protect against it, there are many. To protect the metal from rust, industrial methods are used. In domestic conditions, various silicone enamels, varnishes, paints, polymeric materials are used.
Industrial
The protection of iron from corrosion can be divided into several main areas. Corrosion protection methods:
- Passivation. Upon receipt of steel, other metals are added (chromium, nickel, molybdenum, niobium and others). They are characterized by high quality characteristics, refractoriness, resistance to aggressive media, etc. As a result, an oxide film is formed. Such types of steel are called alloyed.

- Surface coating with other metals. Different methods are used to protect metals from corrosion: electroplating, immersion in a molten composition, application to the surface using special equipment. As a result, a metallic protective film is formed. Chromium, nickel, cobalt, aluminum and others are most often used for these purposes. Alloys (bronze, brass) are also used.

- The use of metal anodes, protectors, more often from magnesium alloys, zinc or aluminum. As a result of contact with the electrolyte (water), an electrochemical reaction begins. The protector breaks down and forms a protective film on the steel surface. This technique has proven itself well for the subsea parts of ships and offshore drilling rigs.

- Acid pickling inhibitors. The use of substances that reduce the level of environmental impact on the metal. They are used for conservation, storage of products. And also in the oil refining industry.

- Corrosion and protection of metals, bimetals (cladding). This coating of steel is a layer of another metal or a composite composition. Under the influence of pressure and high temperatures, diffusion and bonding of surfaces occur. For example, well-known bimetal heating radiators.

Corrosion of metal and methods of protection against it, used in industrial production, are quite diverse, these are chemical protection, glass enamel coating, enameled products. Steel is hardened at high, over 1000 degrees, temperatures.
In the video: galvanizing metal as protection against corrosion.
household
Protecting metals from corrosion at home is, first of all, chemistry for the production of paints and varnishes. The protective properties of the compositions are achieved by combining various components: silicone resins, polymer materials, inhibitors, metal powder and shavings.
To protect the surface from rust, it is necessary to use special primers or a rust converter before painting, especially on older structures.

What are the types of converters?
- Primers - provide adhesion, adhesion to metal, level the surface before painting. Most of them contain inhibitors that significantly slow down the corrosion process. Preliminary application of a primer layer can significantly save paint.
- Chemical compounds - convert iron oxide into other compounds. They are not subject to rust. They are called stabilizers.
- Compounds that convert rust into salts.
- Resins and oils that bind and seal rust, thus neutralizing it.
The composition of these products includes components that slow down the process of rust formation as much as possible. Converters are included in the product line of manufacturers producing paints for metal. They are different in terms of their texture.
It is better to choose a primer and paint from the same company so that they are suitable in terms of chemical composition. First you need to decide which methods you will choose to apply the composition.

Protective paints for metal
Paints for metal are divided into heat-resistant, which can be operated at high temperatures, and for ordinary temperature regime up to eighty degrees. The following main types of paints for metal are used: alkyd, acrylic, epoxy paints. There are special anti-corrosion paints. They are two- or three-component. They are mixed immediately before use.
Advantages of paintwork for metal surfaces:
- well protect surfaces from temperature changes and atmospheric fluctuations;
- quite easily applied in different ways (brush, roller, using an airbrush);
- most of them are quick-drying;
- wide range of colors;
- long operating periods.

Of the available inexpensive means, you can use the usual silver. It contains aluminum powder, which creates a protective film on the surface.

Epoxy two-component compounds are suitable for protecting metal surfaces that are subjected to increased mechanical stress, in particular the underbody of cars.
Metal protection at home
Corrosion, methods of protection against it in domestic conditions require compliance with a certain sequence:
1. Before applying a primer or rust converter, the surface is thoroughly cleaned of dirt, oil stains, rust. Use metal brushes or special attachments for grinders.

2. Then a primer layer is applied, allowed to soak and dry.


Protecting metals from corrosion is a complex process. It begins at the stage of steel smelting. It is difficult to list all the rust control methods, as they are constantly being improved, not only in industry, but also for domestic use. Manufacturers of paints and varnishes are constantly improving the compositions, increasing their corrosive properties. All this significantly extends the service life of metal structures and steel products.
INTERSTATE STANDARD
Unified system of protection against corrosion and aging
METALS AND ALLOYS
Methods of determination
corrosion indicators
and corrosion resistance
GOST 9.908-85
MOSCOW
IPK STANDARDS PUBLISHING HOUSE
1999
INTERSTATE STANDARD
Introduction date 01.01.87
This standard establishes the main indicators of corrosion and corrosion resistance (chemical resistance) of metals and alloys with continuous, pitting, intergranular, exfoliating corrosion, spot corrosion, corrosion cracking, corrosion fatigue and methods for their determination. Indicators of corrosion and corrosion resistance are used in corrosion research, testing, inspection of equipment and fault detection of products during production, operation, storage.
1. INDICATORS OF CORROSION AND CORROSION RESISTANCE
1.1. The indicators of corrosion and corrosion resistance of the metal are determined under given conditions, taking into account their dependence on the chemical composition and structure of the metal, the composition of the medium, temperature, hydro- and aerodynamic conditions, the type and magnitude of mechanical stresses, as well as the purpose and design of the product. 1.2. Corrosion resistance indicators can be quantitative, semi-quantitative (point) and qualitative. 1.3. Corrosion resistance should, as a rule, be characterized by quantitative indicators, the choice of which is determined by the type of corrosion and operational requirements. The basis of most of these indicators is the time to reach a given (permissible) degree of corrosion damage to the metal under certain conditions. Corrosion resistance indicators, primarily the time until the permissible depth of corrosion damage is reached, in many cases determine the service life, durability and shelf life of structures, equipment and products. 1.4. The main quantitative indicators of corrosion and corrosion resistance of the metal are given in the table. For a number of corrosion effects (integral corrosion indicators), the corresponding speed (differential) corrosion indicators are given.|
Type of corrosion |
The main quantitative indicators of corrosion and corrosion resistance |
||
|
Corrosion effect (integral corrosion index) |
Speed (differential) corrosion index |
Corrosion resistance index |
|
| continuous corrosion | Corrosion penetration depth | Linear corrosion rate | Corrosion penetration time to the allowable (given) depth* |
| Mass loss per unit area | Weight loss rate | Time to reduce the mass by an allowable (specified) value * | |
| stain corrosion | Degree of damage to the surface | ||
| Pitting corrosion | Maximum Pitting Depth | Max Speed pitting penetration | Minimum pit penetration time to allowable (specified) depth* |
| Maximum diameter of pitting at the mouth | The minimum time to reach the allowable (specified) size of the diameter of the pitting at the mouth * | ||
| The degree of damage to the surface by pitting | Time to reach the permissible (specified) degree of damage * | ||
| Intergranular corrosion | Penetration time to allowable (specified) depth* | ||
| Decreased mechanical properties (relative elongation, narrowing, impact strength, tensile strength) | Time to reduce the mechanical properties to an acceptable (specified) level* | ||
| stress corrosion cracking | Depth (length) of cracks | crack growth rate | Time to first crack** |
| Decreased mechanical properties (relative elongation, narrowing) | Time to sample failure** Level of safe stresses** (conditional limit of long-term corrosion strength**) Threshold stress intensity factor for corrosion cracking** | ||
| Corrosion fatigue | Depth (length) of cracks | crack growth rate | Number of cycles before specimen failure** Conditional corrosion fatigue limit** Threshold stress intensity factor for corrosion fatigue** |
| exfoliating corrosion | The degree of damage to the surface by delaminations The total length of the ends with cracks | ||
| Corrosion penetration depth | Corrosion penetration rate | ||
Scheme of dependence of the corrosion effect (integral index) at from time
1.6. It is allowed to use, along with the indicators given in the table, other quantitative indicators determined by operational requirements, high sensitivity of experimental methods or the possibility of using them for remote monitoring of the corrosion process, with a preliminary establishment of the relationship between the main and applied indicators. As such indicators of corrosion, taking into account its type and mechanism, the following can be used: the amount of hydrogen released and (or) absorbed by the metal, the amount of oxygen reduced (absorbed), an increase in the mass of the sample (while maintaining solid corrosion products on it), a change in the concentration of corrosion products in medium (with their complete or partial solubility), an increase in electrical resistance, a decrease in reflectivity, a decrease in heat transfer coefficient, a change in acoustic emission, internal friction, etc. For electrochemical corrosion, it is allowed to use electrochemical indicators of corrosion and corrosion resistance. In case of crevice and contact corrosion, the corrosion and corrosion resistance indicators are selected from the table in accordance with the type of corrosion (solid or pitting) in the crevice (gap) or contact zone. 1.7. For one type of corrosion, it is allowed to characterize the results of corrosion tests by several corrosion indicators. In the presence of two or more types of corrosion on one sample (product), each type of corrosion is characterized by its own indicators. Corrosion resistance in this case is evaluated by an indicator that determines the performance of the system. 1.8. If it is impossible or inappropriate to determine quantitative indicators of corrosion resistance, it is allowed to use qualitative indicators, for example, a change in the appearance of the metal surface. At the same time, the presence of tarnishing is visually established; corrosion damage, the presence and nature of the layer of corrosion products; the presence or absence of an undesirable change in the environment, etc. On the basis of a qualitative indicator of corrosion resistance, an assessment is made of the type: resistant - not resistant; good - not good, etc. A change in appearance is allowed to be assessed by points on conditional scales, for example, for electronic equipment products in accordance with GOST 27597. 1.9. Permissible indicators of corrosion and corrosion resistance are set in the regulatory and technical documentation for the material, product, equipment.
2. DETERMINATION OF CORROSION INDICATORS
2.1. Continuous corrosion 2.1.1. Mass loss per unit surface area D m, kg / m 2, calculated by the formulaWhere m 0 - mass of the sample before testing, kg; m 1 - mass of the sample after testing and removal of corrosion products, kg; S- sample surface area, m 2 . 2.1.2. When hard-to-remove solid corrosion products are formed or their removal is inexpedient quantification continuous corrosion is carried out by increasing the mass. The increase in mass per unit surface area is calculated from the difference in the masses of the sample before and after testing, referred to the unit surface area of the sample. To calculate the mass loss of the metal by increasing the mass of the sample, it is necessary to know the composition of the corrosion products. This indicator of metal corrosion in gases at high temperature is determined according to GOST 6130. 2.1.3. Corrosion products are removed according to GOST 9.907. 2.1.4. The change in dimensions is determined by direct measurements from the difference between the dimensions of the sample before and after testing and removal of corrosion products. If necessary, change the dimensions according to the loss of mass, taking into account the geometry of the sample, for example, changing the thickness of a flat sample D L, m, calculated by the formula
Where D m- weight loss per unit area, kg/m 2 ; ρ is the density of the metal, kg/m 3 . 2.2. Spot corrosion 2.2.1. The area of each spot is determined with a planimeter. If such a measurement is not possible, the spot is outlined by a rectangle and its area is calculated. 2.2.2. The degree of damage to the metal surface by corrosion spots ( G) as a percentage is calculated by the formula
Where Si- square i-th spot, m 2; n - the number of spots; S - sample surface area, m 2 . It is allowed to determine the degree of damage to the surface by corrosion with the help of a grid of squares in case of corrosion with spots. 2.3. Pitting corrosion 2.3.1. The maximum penetration depth of pitting corrosion is determined by: measuring the distance between the mouth plane and the bottom of the pitting with a mechanical indicator with a movable needle probe after removing corrosion products in cases where the dimensions of the pitting allow free penetration of the needle probe to its bottom; microscopically, after removal of corrosion products by measuring the distance between the mouth plane and the bottom of the pit (double focusing method); microscopically on a transverse section at an appropriate magnification; successive mechanical removal of metal layers of a given thickness, for example, by 0.01 mm until the last pits disappear. Pittings with a mouth diameter of at least 10 µm are taken into account. The total area of the working surface must be at least 0.005 m 2 . 2.3.2. A section for measuring the maximum penetration depth of pitting corrosion is cut out from the area where the largest pittings are located on the working surface. The cut line should pass through as many of these pits as possible. 2.3.3. The maximum penetration depth of pitting corrosion is found as the arithmetic mean of measurements of the deepest pittings depending on their number ( n) on the surface: at n < 10 измеряют 1-2 питтинга, при n < 20 - 3-4, при n> 20 - 5. 2.3.4. With through pitting corrosion, the thickness of the sample is taken as the maximum penetration depth. 2.3.5. The maximum diameter of the pitting is determined using measuring instruments or optical means. 2.3.6. The degree of damage to the metal surface by pitting is expressed as a percentage of the surface occupied by pitting. In the presence of a large number of pits with a diameter of more than 1 mm, it is recommended that the degree of damage be determined according to clause 2.2. 2.4. Intergranular corrosion 2.4.1. The depth of intergranular corrosion is determined by the metallographic method according to GOST 1778 on an etched section made in the transverse plane of the sample, at a distance from the edges of at least 5 mm at a magnification of 50 ´ or more. It is allowed to determine the penetration depth of aluminum corrosion and aluminum alloys on unetched sections. Etching mode - according to GOST 6032, GOST 9.021 and NTD. (Revised edition, Rev. No. 1). 2.4.2. The change in mechanical properties during intergranular corrosion - tensile strength, relative elongation, impact strength - is determined by comparing the properties of metal samples that have been subjected to and not subjected to corrosion. The mechanical properties of metal samples that have not undergone corrosion are taken as 100%. 2.4.3. Samples are made in accordance with GOST 1497 and GOST 11701 when determining the tensile strength and relative elongation, and according to GOST 9454 - when determining impact strength. 2.4.4. It is allowed to use physical methods for controlling the depth of corrosion penetration in accordance with GOST 6032. 2.5. Corrosion cracking and corrosion fatigue 2.5.1. In corrosion cracking and corrosion fatigue, cracks are detected visually or using optical or other flaw detection tools. It is allowed to use indirect measurement methods, for example, determining the increase in the electrical resistance of the sample. 2.5.2. The change in mechanical properties is determined according to clause 2.4.2. 2.6. Exfoliating corrosion 2.6.1. The degree of surface damage during exfoliating corrosion is expressed as a percentage of the area with peeling on each surface of the sample according to GOST 9.904. 2.6.2. The total length of the ends with cracks for each sample ( L) as a percentage is calculated by the formula
Where L i- length of the end section affected by cracks, m; P- sample perimeter, m. 2.6.3. It is allowed to use the conditional scale score according to GOST 9.904 as a generalized semi-quantitative (point) indicator of exfoliating corrosion.
3. DETERMINATION OF INDICATORS OF CORROSION RESISTANCE
3.1. Continuous corrosion 3.1.1. The main quantitative indicators of corrosion resistance against continuous corrosion in the absence of special requirements, for example, in terms of environmental pollution, are determined from the table. 3.1.2. When continuous corrosion occurs at a constant rate, the corrosion resistance indicators are determined by the formulas:Where tm- time to decrease in mass per unit area by admissible value D m, year; v m- weight loss rate, kg / m 2 ∙ year; t 1 - penetration time to the allowable (given) depth ( l), year; v 1 - linear corrosion rate, m/year. 3.1.3. When continuous corrosion occurs at a non-constant rate, the corrosion resistance indicators are determined according to clause 1.5. 3.1.4. If there are special requirements for the optical, electrical and other properties of the metal, its corrosion resistance is estimated by the time of change of these properties to an acceptable (specified) level. 3.2. Stain Corrosion The index of corrosion resistance in spot corrosion is the time (t n) to achieve an acceptable degree of damage to the surface. t value n determined graphically according to clause 1.5. 3.3. Pitting corrosion 3.3.1. The main indicator of corrosion resistance against pitting corrosion is the absence of pitting or the minimum time (t pit) for penetration of pitting to an allowable (given) depth. t pit is determined graphically from the dependence of the maximum pitting depth l max from time. 3.3.2. An indicator of resistance to pitting corrosion can also serve as the time to reach an acceptable degree of damage to the surface by pitting. 3.4. Intercrystalline corrosion 3.4.1. Indices of corrosion resistance against intergranular corrosion are generally determined graphically or analytically from the time dependence of the penetration depth or mechanical properties in accordance with clause 1.5. 3.4.2. A qualitative assessment of resistance against intergranular corrosion of the type of racks - not racks based on accelerated tests of corrosion-resistant alloys and steel is established according to GOST 6032, aluminum alloys - according to GOST 9.021. 3.5. Corrosion cracking 3.5.1. Quantitative indicators of resistance to corrosion cracking are determined for high-strength steels and alloys according to GOST 9.903, for aluminum and magnesium alloys - according to GOST 9.019, welded joints steel, copper and titanium alloys - according to GOST 26294-84. 3.6. Exfoliating corrosion 3.6.1. The indicators of resistance to exfoliating corrosion for aluminum and its alloys are determined according to GOST 9.904, for other materials - according to NTD.
4. PROCESSING THE RESULTS
4.1. It is recommended to carry out pre-processing results in order to identify abnormal (outliers) values. 4.2. The dependence of the corrosion effect (integral corrosion index) on time in the case of its monotonous change is recommended to be expressed graphically, using at least four index values for plotting. 4.3. The results of the calculation of corrosion and corrosion resistance indicators are recommended to be expressed as a confidence interval of the numerical value of the indicator. 4.4. The regression equation, confidence intervals and accuracy of the analysis are determined according to GOST 20736, GOST 18321. 4.5. The metallographic method for assessing corrosion damage is given in Appendix 1. (Introduced additionally, Rev. No. 1).APPENDIX.(Deleted, Rev. No. 1).ATTACHMENT 1
Mandatory
METALLOGRAPHIC METHOD FOR ASSESSING CORROSION DAMAGES
1. The essence of the method
The method is based on determining the type of corrosion, the form of corrosion damage, the distribution of corrosion damage in metals, alloys and protective metal coatings (hereinafter referred to as materials) by comparing with the corresponding standard forms, as well as measuring the depth of corrosion damage on a metallographic section.
2. Samples
2.1. The location of sampling from the material under test is selected based on the results of visual (with the naked eye or with a magnifying glass) inspection of the surface or non-destructive flaw detection. 2.2. Samples are cut from the following places in the material: 1) if only part of the surface of the material is affected by corrosion, samples are taken in three places: from the part affected by corrosion; from a part not affected by corrosion, and in the area between them; 2) if there are areas of the surface of the material with various types corrosion or with different depth of corrosion damage, samples are taken from all areas affected by corrosion; 3) if there is one type of corrosion damage on the surface of the material, samples are taken from at least three characteristic areas of the material under study. 2.3. If necessary, at least one sample is taken from at least five functionally necessary sections of the test material. The size of the sample is determined based on the size of the zone of corrosion damage. 2.4. Samples are cut in such a way that the plane of the section is perpendicular to the surface under study. The manufacturing method should not affect the structure of the material and destroy the surface layer and edges of the sample. For materials with protective coatings, damage to the coating and its separation from the base material is not allowed. 2.5. Sample marking - according to GOST 9.905. 2.6. In the manufacture of a metallographic section, all traces of cutting, for example, burrs, are removed from the surface of the sample. 2.7. When grinding and polishing the section, it is necessary to ensure that the nature and size of the corrosion damage does not change. The edges of the section in the place of corrosion damage should not have roundings. Roundings are allowed that do not affect the accuracy of determining the corrosion damage. To do this, it is recommended to pour the sample into the casting mass in such a way that the edge under study is at a distance of at least 10 mm from the edge of the section. Polishing is carried out for a short time using diamond pastes. 2.8. Evaluation of the section is carried out before and after etching. Etching makes it possible to distinguish between corrosion damage and the structure of the material. When pickling, the nature and size of the corrosion damage should not be changed.
3. Testing
3.1. Determination and assessment of the type of corrosion, the form of corrosion damage and its distribution in the material 3.1.1. The test shall take into account the chemical composition of the material being tested, the method of its processing, as well as any corrosive factors. 3.1.2. The test is carried out on a metallographic section under a microscope at a magnification of 50, 100, 500 and 1000 ´ . 3.1.3. When determining the type of corrosion, the control of corrosion damage is carried out along the entire length of the section. It is allowed to determine several types of corrosion on one sample. 3.1.4. When testing protective coatings, the determination of the type of corrosion of the coating and the base material is carried out separately. 3.1.5. If the material, in addition to the corrosive environment, is also affected by other factors that affect the change in the structure of the material, for example, high temperature, mechanical stress, corrosion damage is determined by comparing the material with a specific sample subjected to the influence of similar factors, but protected from the impact of a corrosive environment. 3.1.6. Evaluation of the form of corrosion damage and determination of the type of corrosion is carried out by comparison with typical schemes of corrosion damage according to Appendix 2, the distribution of corrosion damage in the material - according to Appendix 3. 3.2. Measuring the depth of corrosion damage 3.2.1. The depth of corrosion damage is determined on a micrometallographic section using an ocular scale and a micrometer screw of a microscope. 3.2.2. The depth of corrosion damage is determined by the difference in the thickness of the metal of the corroded section of the surface of the section and the surface area without corrosion or by measuring the depth of damage from the surface that is not destroyed or slightly destroyed by corrosion. When testing a material with a protective coating, the results of measuring the depth of corrosion damage to the coating and the base metal are determined separately. 3.2.3. If the entire surface of the sample is affected by corrosion and the depth of corrosion damage in different parts of the surface does not noticeably differ, for example, in the case of intergranular or transgranular corrosion, the depth of corrosion damage is measured in at least 10 areas of the surface. For large samples, measurements are taken at least in 10 areas for every 20 mm of the length of the inspected surface, taking into account the deepest lesions. 3.2.4. In the case of local corrosion damage (for example, pitting corrosion or spotting corrosion), measurements are carried out at the places of this corrosion damage, and the number of measurement sites may differ from the requirements given in paragraph 1. 3.2.3. 3.2.5. To clarify the determination of the maximum depth of corrosion damage after the metallographic assessment of sections, they are re-grinded: until the moment when the measured depth is less than the previous measurement result; 2) for samples with almost the same depth of corrosion damage in different parts of the surface, after evaluation, regrinding is carried out and a new metallographic section is made, on which corrosion damage is again assessed. 3.2.6. The error in measuring the depth of corrosion damage is no more than ±10%.
4. Test report - according to GOST 9.905
ATTACHMENT 1.(Introduced additionally, Amendment No. 1).APPENDIX 2
Mandatory
TYPES OF CORROSION
|
Type of corrosion |
Characteristics of the form of corrosion damage |
Scheme of a typical type of corrosion damage |
| 1. Solid (uniform) corrosion | Forms of corrosion damage 1a and 1b differ only in surface roughness. By changing the shape of the surface before and after the corrosion test, the presence of corrosion is detected: it is determined by the change in the mass and dimensions of the samples before and after the corrosion test |
|
| Form 1c can be transitional between continuous and selective corrosion, for example, 10c, 10d and 10e The type of corrosion can be specified by changes in its shape depending on the time of exposure to the corrosive environment, as well as by the structure of the metal |
|
|
| 2. Local (uneven) corrosion | The shape corresponds to continuous corrosion, but differs in that part of the surface is subject to corrosion or corrosion proceeds at different rates in its individual sections. | |
| 3. Corrosion stains | Minor corrosion damage of irregular shape; the size of its area in the case of a small increase may exceed the size of the field of view |
|
| 4. Corrosion pit | Corrosion damage with a depth approximately equal to the width |
|
| 5. Pitting corrosion | Corrosion damage with a depth significantly greater than the width |
|
| 6. Subsurface corrosion | Corrosion damage, characterized by the fact that it occupies a small area on the surface and is mainly concentrated under the surface of the metal |
|
|
|
||
| A form of corrosion damage in which individual zones are below the surface and usually do not have a noticeable direct exit to the surface. |
|
|
| 7. Layered corrosion | Corrosion damage, the inner layers of which include grains of various sizes, various phases, inclusions, segregations, etc. | |
| 8. Intergranular corrosion | Corrosion damage is characterized by the presence of a corroded zone along the boundaries of metal grains, and it can affect the boundaries of all grains or only individual grains. |
|
| 9. Transcrystalline corrosion | Corrosion damage is characterized by the presence of a large number of transcrystalline cracks. |
|
| 10. Selective corrosion | Corrosion damage to which a certain structural phase or component is subjected; if the phase is formed by a eutectic, it is determined whether the whole eutectic or some of its components, for example, cementite, is corroded |
|
| Corrosion damage to which a certain phase of the metal is subjected without direct contact with the corroded surface. In this case, it is determined whether the phases corrode along the grain boundaries or within the grains of the main structure. Next, it is determined whether the boundaries between the corroding phases differ from the rest of the boundaries (the presence of a phase, cracks). From this it is concluded whether the corrosive medium penetrates along the grain boundaries or diffuses through the entire volume of the grains | ||
| Corrosion damage to which only individual grains are subjected, the physical state of which has changed, for example, due to deformation |
|
|
| Corrosion damage to which only the deformable parts of the grains are subjected, while the resulting corrosion damage zone is narrower than one grain and passes through several grains. At the same time, it is determined whether the deformation has affected the change in the structure of the metal, for example, the transition of austenite to martensite |
|
|
| Corrosion damage in the form of a zone with rows of isolated inclusions; at the same time, a possible change in the structure in this zone is determined |
|
|
| Corrosion damage in the form of a wide zone along the grain boundary. This form may be temporary and cannot be attributed to intergranular corrosion; it is characterized by the fact that it does not penetrate into the depth of the metal. More precisely, it can be determined by changes in the form of corrosion damage depending on the time of corrosion exposure and by the release of structural particles in a corroding alloy. | ||
| Corrosion damage, which results in the formation of a new phase of the metallic appearance, which has the ability to reduce the resistance of the metal | ||
| Corrosion damage, as a result of which the chemical composition of the phase changes while maintaining its shape and location, for example, graphitization of cementite plates in cast iron, dezincification of brass, etc. Other corrosion products, for example, oxides, can form in the zone of this change. |
|
|
| 11. Corrosion in the form of rare cracks | Corrosion damage, which results in the formation of a deep, slightly branched crack, wide near the surface with a gradual transition to a slight width; crack filled with corrosion products |
|
| Corrosion damage in the form of a deep crack of insignificant width emanating from a corrosion pit on the surface; the crack may have a branched shape |
|
|
| Corrosion damage, as a result of which an intergranular crack of insignificant width is formed in the absence of corrosion products. Compared to intergranular corrosion, it has the form of single (rare) cracks |
|
|
| Corrosion damage, as a result of which a transcrystalline crack of insignificant width with significant branching is formed. Compared to transcrystalline corrosion, it has the form of single (rare) cracks. Some cracks may be partly transgranular and partly intergranular. | ||
| Corrosion damage, as a result of which cracks of insignificant width are formed, having the form of threads, mainly parallel to the surface and creating a zone of a certain depth. They cannot be attributed to similar cracks formed due to deformation or poor processing of the sample. |
|
|
| Corrosion damage in the form of small, predominantly short cracks inside individual grains. Cracks can be formed, for example, due to the action of molecular hydrogen, high stress, corrosion of a certain phase |
APPENDIX 3
Mandatory
DISTRIBUTION OF CORROSION
APPENDIX 3(Introduced additionally, Amendment No. 1).INFORMATION DATA
1. DEVELOPED AND INTRODUCED by the USSR State Committee for Product Quality Management and StandardsDEVELOPERSL.I. Topchiashvili, G.V. Kozlova, cand. tech. sciences (topic leaders); V.A. Atanova, G.S. Fomin, cand. chem. Sciences, L.M. Samoilova, I.E. Trofimova 2. APPROVED AND INTRODUCED BY Decree of the USSR State Committee for Standards dated October 31, 1985 No. 3526 3. The standard fully complies with ST SEV 4815-84, ST SEV 6445-88 4. INTRODUCED FOR THE FIRST TIME 5. REFERENCE REGULATIONS AND TECHNICAL DOCUMENTS|
Item number, applications |
Item number, applications |
||
| GOST 9.019-74 | 3.5.1 | GOST 6032-89 | 2.4.1; 2.4.4; 3.4.2 |
| GOST 9.021-74 | 2.4.1; 3.4.2 | GOST 6130-71 | 2.1.2 |
| GOST 9.903-81 | 3.5.1 | GOST 9454-78 | 2.4.3 |
| GOST 9.904-82 | 2.6.1; 2.6.3; 3.6.1 | GOST 11701-84 | 2.4.3 |
| GOST 9.905-82 | Attachment 1 | GOST 18321-73 | 4.4 |
| GOST 9.907-83 | 2.1.3 | GOST 20736-75 | 4.4 |
| GOST 1497-84 | 2.4.3 | GOST 26294-84 | 3.5.1 |
| GOST 1778-70 | 2.4.1 | GOST 27597-88 | 1.8 |
Corrosion protection system: how and why?
The disadvantage of a material such as metal is that corrosion can occur on it. To date, there are several methods, they need to be used in combination. The corrosion protection system will help get rid of rust and prevent the formation of layers.
Treating a metal surface with a special coating is an effective way. Metal coating increases the hardness and strength of the material, improves mechanical properties. It must be taken into account that in this case additional protection. Non-metallic coating is applied to ceramics, rubber, plastic, wood.
Corrosion protection methods
Most often, film-forming coatings are used, they are resistant to external environment. A film forms on the surface, which inhibits corrosion processes.
In order to reduce corrosivity, it is necessary to neutralize the environment affected by it. Inhibitors will help you with this, they are introduced into an aggressive environment, and a film is formed that slows down the processes and changes the chemical parameters of the metal.
Alloying is widely used, it enhances properties that help increase the resistance of the material to corrosive processes. Alloy steel contains a lot of chromium in its composition, it forms films that protect the metal.
It will not be superfluous to use protective films. Anode coatings are used for zinc and chromium, cathodic coatings for tin, nickel, and copper. They are applied using a hot method, galvanization can also be used. The product must be placed in a container in which the protective metal is in a molten state.
Using plating, corrosion can be avoided. The surface is covered with a metal in a molten state, it is sprayed with air. The advantage of this method is that it can cover finished and fully assembled structures. The downside is that the surface will be a little rough. Such coatings are applied by diffusion into the metal that is the main one.
The coating can be protected with an oxide film, this procedure is called oxidation. The oxide film that is on the metal is treated with a powerful oxidizing agent, as a result of which it becomes several times stronger.
Phosphating is also used in industry. Iron salts are immersed in a hot solution of phosphates, eventually forming a surface film.
For temporary protection of the surface, it is necessary to use ethinol, technical vaseline, inhibitors. The latter slow down the reaction, as a result of which corrosion develops much more slowly.



























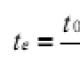


For beginners: breeding a broiler at home Boiled water for broilers
Only lovers will survive
Features of advertising aimed at children
retouching old photos in photoshop retouching old photos
What is an NPO: decoding, definition of goals, types of activities Does a non-profit organization have the right