
Lesson objectives:
- To concretize and deepen the knowledge of schoolchildren about
variety and importance of fertilizers, their classification,
Nomenclature.
2. Develop the skills of conducting a chemical experiment to study the properties of substances.
3. To introduce the professions of agricultural workers.
4. Develop computational skills to find the mass fraction of a chemical element in a substance.
5. To form the skills of rational land use.

During the classes:
- Organizing time.
2. Actualization of knowledge.
3. Learning new material.
4. Solution of design problems for calculating the mass fraction of a chemical element in a substance.
5. Laboratory work Introduction to nitrogen and phosphate fertilizers.
6. 6. Acquaintance with the professions of agriculture.
7. Generalization.
8. The results of the lesson.

Chemical elements in plants.
Table 1. The content of chemical
elements in a cell
Element
Quantity, %
Oxygen


fertilizers
organic
mineral

organic fertilizers
Manure
slurry
Compost
Biohumus


In the practice of agriculture, green manure has been used since time immemorial. In Europe, this technique, borrowed from China, began to spread in the Mediterranean countries already in the days of Ancient Greece. Here, by the way, it would be appropriate to quote the words of the Roman writer and scientist Pliny the Elder, who lived in 23-79 AD. With regard to green fertilizer, Pliny says the following: "Everyone agrees that there is nothing more useful than lupine, if it is planted in the soil with a plow before the formation of beans, or bunches of lupine, cut off at the surface of the soil, are buried near the roots of fruit trees and grape bushes ... This is the same good fertilizer, like manure."

mineral
fertilizer
Phosphoric
Potash
Nitrogen
Microfertilizers

culture
Yield increase from application
1 centner of nitrogen fertilizer
4-5 centner
Sugar beet
30-40 centner
potato
20-30 centner

Removal of batteries with the harvest
culture
Products
Removal per 1 ton of harvest, kg
Wheat
winter
Yarovaya
P 2 O 5
corn
Rye
corn
K 2 O
Corn
corn
corn
oats
corn
Barley
corn
Peas
corn
Len-fibre
seeds,
fiber
Sugar beet
Roots
Potato
tubers
clover red
Hay
Approximate coefficients of assimilation of nutrients
fertilizers
Fertilizer utilization rates, %
mineral
organic
P 2 O 5
K 2 O

Batteries
Signs of deficiency
Leaves are small, pale green and yellow
The leaves are bluish, dark green, with a reddish tint, the drying leaves are dark.
The leaves are yellow or brown, wrinkled, twisted along the edges.
The lack of chemical elements causes metabolic disorders in plants
Batteries
Signs of deficiency
The death of growth points in plants; flax and cotton fall off buds, flowers, ovaries; in sugar beets, the core rots and a hollow root forms.
The tips of the leaves turn white, the leaves dry up, the seeds do not set.
Manganese
In grain breads, the leaves turn yellow, spotting occurs with white and brown stripes.

Acute poisoning with nitrates and nitrites.
Nitrate ions are converted in the human body into nitrite ions, which cause the disease methylobeanemia (hemoglobin interacts with NO2‾ and loses the ability to carry oxygen). In the intestinal tract, nitrites are converted into nitrosamines, which are strong carcinogenic agents.
Permissible norms of nitrates for humans.

AT agriculture ash is used as potassium-phosphorus and lime fertilizer. It contains all the main nutrients (phosphorus, potassium, calcium), except for nitrogen (it volatilizes during combustion). There are about 30 trace elements in the ash. Including magnesium, sulfur, iron, boron, manganese, etc. All of them are in a form available to plants.
Component name
CaCO 3
CaSiO 3
NaPO 4
CaSO 4
K 3 PO 4
CaCl 2
MgCO 3
MgSiO 3
MgSO 4
Ash helps in the fight against some small sucking pests. She is dusted with seedlings of radish, radish, potatoes and other crops. She saves the cabbage from the defeat of the keel.
Use ashes - and you will see how your plants will change, prettier!

A task.
How much potassium nitrate you need to take to grow 1 ton of potatoes,
if the removal of nitrogen is 5 kg., the assimilation rate is 60%.
Given:
M (potatoes) = 1 ton
Find: m(K NO 3)?
Solution:
1. Calculate the mass fraction of nitrogen in potassium nitrate.
M(K NO 3)=39+14+16x3=101 g/mol
M( N )=14 g/mol
101 g/mol - 100%
14 g / mol - x%
X=14x100:101=13.6%
2. Find the mass of nitrogen
5kg.-60%
X kg. - 100%
X \u003d 5x100: 60 \u003d 8.33 kg.
3. Find the mass K NO 3.
8.33kg. – 13.6%
X kg. - 100%
X \u003d 8.33x100: 13.6 \u003d 61.25 kg.
Answer: m(K NO 3)=61.25 kg.

The amount of fertilizer applied depends on the chemical composition of the soil, which is determined by soil analysis in an agrochemical laboratory.
The need for a chemical element = the content of the element in the soil + the content of the chemical element in the fertilizer.




Laboratory work
"Familiarization with samples of nitrogen, potash and phosphorus fertilizers".
Progress.
- Review the fertilizer samples given to you. Describe them appearance, study the solubility in water.
- Present the results of your work in the form of a table.
Fertilizer name
Chemical formula
Appearance
Solubility in water




Rules for rational land use:
- Application of crop rotation.
- Proper digging in spring and autumn.
- Application of fertilizers.
- Snow retention.

List of used literature:
- Ustimenko G.V., Shcherbakov M.I. Agrotechnics of field crops Tutorial for students in grades 9-10 of a rural school. Moscow: Education, 1978.
- Klepinina Z.A. And others. Labor training. Agricultural work. Textbook for students in grades 5-17 of a rural school. Moscow: Education, 1989.
- Korchagina V.A. Biology: bacteria, fungi, lichens: a textbook for grades 6-7 environments. school –M.: Enlightenment, 1993.
- Zhidkin V.A. Fundamentals of ecology. Textbook for grades 10-11 high school. Saransk book publishing house, 1995.
List of Internet resources used:
- http://shola/iv/index/php/
- http://window/edu. en/
- http://ovoport. en/
- http://wikipedia.g u
- http:// wikisource. org.

1.Chemical composition plants.
2. Definition of what is "mineral fertilizers".
3. Classification of mineral fertilizers.
4. Requirements for mineral fertilizers.
5. Solving problems for the recognition of fertilizers.

one). The chemical composition of plants.
The chemical composition of plants.
Macronutrients Micronutrients
N, P, K, C, S, Mg ,Ca Fe, Cu, Mn, Zn, Co

2). Definition "Mineral fertilizers".
Substances containing three essential nutrients N, P, K and capable of dissociating into ions in the soil solution are used as mineral fertilizers.

3) Classification of mineral fertilizers.
Simple Complex

4). Requirements for mineral fertilizers:
A) contains a large% of the nutrient;
B) must be soluble in water;
C) better in granules if hygroscopic
D) it is better if it is complex.

5). How to distinguish fertilizers from each other?
Exercise. Make a fertilizer recognition plan:
a) ammonium nitrate and ammonium chloride;
b) ammonium nitrate and ammonium sulfate;
c) potassium chloride and sylvinite;
d) superphosphate from other fertilizers;
e) superphosphate from ammonium chloride.

Fertilizer recognition plan
Fertilizer name
with H 2 S O 4
(conc.) and Cu
ammonium nitrate
ammonium chloride
with BaCI solution 2
brown gas
ammonium nitrate
ammonium sulfate
with NaOH at t 0
brown gas
potassium chloride
sylvinite
Ammonia is released
Ammonia is released
with AgNO solution 3
Ammonia is released
Ammonia is released
Small↓
(from impurities)

Fertilizer recognition plan
Fertilizer name
(conc.) and Cu
Superphos - fat from other fertilizers
with BaCI 2 solution
Superphos - fat
ammonium chloride
with AgNO 3 solution
Ammonia is released
Yellow ↓
Yellow ↓

Description of the presentation on individual slides:
1 slide
Description of the slide:
2 slide
Description of the slide:
Objectives: studying the composition of mineral fertilizers and determining their biological role, classification of fertilizers, formation of skills in solving problems, strengthening the skills of recognizing inorganic substances with the help of qualitative reactions to ions, activation of cognitive interest, broadening the general outlook, developing the ability to apply acquired knowledge in practice.
3 slide
Description of the slide:
Mineral fertilizers are compounds containing nutrients necessary for plants. Plant cells contain more than 70 chemical elements - almost all that are found in the soil. But for the normal growth, development and fruiting of plants, only 16 of them are needed. These are elements absorbed by plants from air and water - oxygen, carbon and hydrogen, and elements absorbed from the soil, among which there are macroelements - nitrogen, phosphorus, potassium, calcium, magnesium, sulfur and trace elements - molybdenum, copper, zinc, manganese , iron, boron and cobalt.
4 slide
Description of the slide:
Individual plants require other chemical elements for normal growth and development. So, for example, sugar beets need sodium to get a high yield of root crops. It also accelerates the growth and improves the development of fodder beet, barley, chicory and other crops. Positive influence Silicon, aluminum, nickel, cadmium, iodine, etc., have an effect on the metabolism in some plants. The needs of agricultural crops for nutrients are most fully satisfied when fertilizers are applied to the soil. No wonder they are figuratively called field vitamins.
5 slide
Description of the slide:
Organo-mineral (ammonia + peat) Organic Manure, compost, peat Mineral Fertilizer classification Nitrogenous Liquid ammonia NH4CI Phosphoric Simple superphosphate Potassium KCI Microfertilizers ZnSO4
6 slide
Description of the slide:
Mineral fertilizers are substances of inorganic origin. According to the active, nutrient element, mineral fertilizers are divided into macro fertilizers: nitrogen, phosphorus, potassium and micro fertilizers (boron, molybdenum, etc.). For the manufacture of mineral fertilizers, natural raw materials (phosphorites, saltpeter, etc.) are used, as well as by-products and wastes of some industries, for example, ammonium sulfate, a by-product in coke chemistry and the production of nylon. Mineral fertilizers are obtained in industry or machining inorganic raw materials, such as grinding phosphorites, or using chemical reactions. They produce solid and liquid mineral fertilizers.
7 slide
Description of the slide:
8 slide
Description of the slide:
Organic fertilizers are substances of plant and animal origin. First of all, it is manure, peat, composts, bird droppings, municipal waste and waste from food production. This includes green fertilizers (lupine plants, beans). Introduced into the soil, these fertilizers decompose under the action of soil microorganisms with the formation of mineral compounds of nitrogen, phosphorus, potassium and other nutrients.
9 slide
Description of the slide:
Organic fertilizers contain organic and mineral substances. They are obtained by treating organic substances (peat, shale, brown coal, etc.) with ammonia and phosphoric acid or by mixing manure or peat with phosphate fertilizers.
10 slide
Description of the slide:
Bacterial fertilizers are preparations (azotobacterin, soil nitragin) containing a culture of microorganisms that absorb soil organic matter and fertilizers and turn them into minerals.
11 slide
Description of the slide:
According to the agrochemical impact, mineral fertilizers are divided into direct and indirect. Direct fertilizers are intended for direct plant nutrition. They contain nitrogen, phosphorus, potassium, magnesium, sulfur, iron and trace elements (B, Mo, Cu, Zn). They are divided into simple and complex fertilizers. Simple fertilizers contain one nutrient (nitrogen, phosphorus, potassium, molybdenum, etc.). These are nitrogen fertilizers, which are distinguished by the form of nitrogen compounds (ammonia, ammonium, amide and combinations thereof); phosphate fertilizers, which are divided into water-soluble (double superphosphate) and insoluble in it (phosphate rock, etc., used on acidic soils); potash fertilizers, which are divided into concentrated (KS1, K2SO3, etc.) and raw salts (sylvinite, kainite, etc.); microfertilizers - substances containing trace elements (H3B03, ammonium molybdate, etc.).
12 slide
Description of the slide:
Complex fertilizers contain at least two nutrients. According to the nature of their production, they are divided into the following groups: mixed - obtained by mechanical mixing of various ready-made powdered or granular fertilizers; complexly mixed granular fertilizers - obtained by mixing powdered ready-made fertilizers with the introduction of liquid fertilizers (liquid ammonia, phosphoric acid, sulfuric acid, etc.) during the mixing process; complex fertilizers - are obtained by chemical processing of raw materials in a single technological process.
13 slide
Description of the slide:
14 slide
Description of the slide:
Indirect fertilizers are used for chemical, physical, microbiological impact on the soil in order to improve the conditions for the use of fertilizers. For example, ground limestone, dolomite, and slaked lime are used to neutralize soil acidity, gypsum is used to improve solonetzes, and sodium hydrosulfite is used to acidify soils. It was agreed to express the nutritional value of fertilizers through the mass fraction of nitrogen N, phosphorus (V) oxide P205 or potassium oxide K20 in them.
15 slide
Description of the slide:
How is the nutrition of plants contained in the soil elements? Let us turn to the theory of electrolytic dissociation. Under the influence of various chemical reactions and with the participation of microorganisms, a gradual transition of nutrients from an indigestible state to an ionic state occurs. But these ions would be washed out by water if they were not retained by soil ion exchangers. The ions retained by ion exchangers make up the bulk of the nutrient materials contained in the soil in a form available to plants. Exchange reactions occur between ion exchangers and solutes.
16 slide
Description of the slide:
Chemical workshop: "Recognition of fertilizers". Materials and equipment: a set of fertilizers, water, solutions of silver nitrate and sodium hydroxide, test tubes, spirit lamp, holder. The following fertilizers are given in three packages under the numbers: 1) ammonium nitrate, 2) phosphate rock, 3) potassium chloride. Experimentally determine which fertilizer is in the bag with the corresponding number. Support your answer with reaction equations. Write full ionic and abbreviated ionic equations.
17 slide
Description of the slide:
Production of mineral fertilizers. Nitrogen fertilizers are produced in factories by binding atmospheric nitrogen with hydrogen. As a result, ammonia is formed, which is then oxidized to nitric acid. By combining ammonia with nitric acid, the most common nitrogen fertilizer is obtained - ammonium nitrate, which contains about 34% nitrogen. An aqueous solution of ammonia containing about 20% nitrogen is used as a fertilizer. Its production is much cheaper than the production of ammonium nitrate. Of the other nitrogen fertilizers, ammonium sulphate is used, containing up to 20% nitrogen, sodium nitrate (16% nitrogen), potassium nitrate (13.5% nitrogen and 46.5% potassium oxide) and urea - the most nitrogen-rich compound (up to 46% nitrogen ). Phosphorous flour is also used as a fertilizer, i.e., finely ground, but not processed chemical phosphorites. The most common potash fertilizer is 40% potassium salt. It occurs naturally as the mineral sylvinite (NaCL*KCL).
18 slide
"Mineral fertilizers" - Phosphorus plays an important role in the life of fruit and berry crops. Production of mineral fertilizers. Nitrogen fertilizers contribute to the development of the green part of the plant. Calculation of the nutritional value of fertilizers. Phosphoric Simple superphosphate, Ca3(PO4)2-phosphorite flour. Nitrogen. Other industries (photochemistry, paint and varnish).
"Chemical industry" - Natural. Synthetic fibers Resins Plastics Rubber Rubber. Chemistry of organic synthesis. Rayon. Polyethylene. Moscow Voronezh Yaroslavl Tolyatti Krasnoyarsk. Carpets. Nitrogen, phosphorus, potassium are biogenic (“life-giving”) elements. Rubber. Ordinary rubbers are produced in Voronezh, Yaroslavl, Tolyatti, Krasnoyarsk.
"Coloring of plastics" - Repair of plastics. durability characteristics. Plastic and environment. Achievements in the field of coloring plastics. Fuel economy. Color selection. What is plastic? Why are plastics used in the automotive industry? Removal of repair parts from the car. Improved comfort.
"Glass" - Quartz glasses have the highest thermal conductivity. Chemical-laboratory glass - glass with high chemical and thermal resistance. Optical glass. In a glassy state, sulfur, selenium, arsenic, and phosphorus can be obtained. Quartz glass. Normal window glass has 0.97W/(m. Thermal conductivity.
"Geography of the chemical industry" - Geography of the chemical industry. Chemical industry. The composition of the industry. In the era of scientific and technological revolution, production continues to grow in the lower floors of the chemical industry, producing sulfuric acid, mineral fertilizers, various pesticides. Growth rates of the chemical industry of the world.
"Production of ammonia" - Tasks. The resulting mixture of NH3, N2, H2 passes through the pipes of the heat exchanger. Raw materials for the production of ammonia. Classification of installations for the synthesis of ammonia. Systems operating at high pressures (450-1000 atm). After passing between the tubes of the heat exchanger, the heated mixture of gases enters the catalyst. The unreacted mixture of N2, H2 enters the synthesis column with the help of a circular compressor.






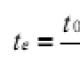



Only lovers will survive
Features of advertising aimed at children
retouching old photos in photoshop retouching old photos
What is an NPO: decoding, definition of goals, types of activities Does a non-profit organization have the right
Gleb Nikitin First Deputy