ELECTROCHEMICAL ENERGY. 2009. V. 9, No. 3. S.161-165
UDC 66.02; 536.7;
METHODS FOR SURFACE TREATMENT OF TITANIUM BIPOLAR PLATES OF HYDROGEN-AIR FUEL CELLS
M. S. Vlaskin, E. I. Shkolnikov, E. A. Kiseleva, A. A. Chinenov*, and V. P. Kharitonov*
Institute of New Energy Problems JIHT RAS, Moscow, Russia *CJSC "Rimos", Moscow, Russia E-mail: [email protected]
Received June 11, 2009
The article is devoted to the study of the influence of surface treatments of bipolar plates (BP) on the specific electrical characteristics of fuel cells (FCs). The studies were carried out on titanium-based plates. Two methods of BP processing are considered: electrochemical gilding and carbon ion implantation. Brief descriptions of the above technologies, as well as the methodology and results of experiments are presented. It is shown that both gold plating and carbon doping of the surface of titanium BPs improve the electrical characteristics of FCs. The relative decrease in FC ohmic resistances compared to uncoated titanium plates was 1.8 for electrochemical gilding and 1.4 for ion implantation.
Keywords Key words: hydrogen-air fuel cells, titanium-based bipolar plates, carbon implantation, impedance spectroscopy.
The Work is dedicated to the research of influence of superficial processings of bipolar plates (BP) on specific electrical characteristics of fuel ce)(s (FC). Researches were conducted on plates on the basis of the titan. Two methods of processing BP are considered: electrochemical gilding and ionic implantation of carbon. In work short descriptions of the resulting technologies, and also a technique and results of experiments are presented. In work it is shown that as gilding, and ionic implantation carbon titanic BP electrical characteristics FC improve. Relative reduction of ohmic resistance FC in comparison with "pure" titanic plates have constituted 1.8 for electrochemical gilding and 1.4 for ionic implantation.
Key words: hydrogen-air fuel cells, bipolar titanium-based plates, carbon implantation, impedance spectroscopy.
INTRODUCTION
Currently, two main types of materials for BP are used in the world: BP from carbon or graphite polymer composites and metal BP.
Research in the field of graphite BP has led to a significant improvement in their physical and chemical properties and specific characteristics. Graphite-based PSUs are more corrosion-resistant than metal ones, but their main disadvantage is still their weak mechanical strength, which prevents their use in fuel cells for transport and portable portable power plants.
In this regard, metals have several undeniable advantages over carbon materials. They are characterized by higher thermal and electrical conductivity, absence of pores, gas impermeability and high mechanical strength. Metal PSUs are also more economical than graphite PSUs. However, all of the above advantages of metals are largely depreciated by such disadvantages as low corrosion resistance and high contact resistance with carbon gas diffusion layers (GDLs).
The most promising metal as a material for the manufacture of power supplies is titanium. The paper presents some advantages of titanium PSUs. Titanium has good mechanical properties, and contamination with titanium ions is not dangerous for the membrane electrode unit (MEA) catalyst. The corrosion resistance of titanium is also one of the highest among metals, however, in the aggressive fuel cell environment, titanium still needs to be protected from corrosion. An additional factor in the search for coatings for titanium is its high contact resistance with carbon HDSs.
Our laboratory (JIHT RAS Laboratory of Aluminum Hydrogen Energy) is engaged in the development of portable power sources based on hydrogen-air fuel cells (HHFC). Titanium was chosen as the BP material, including due to the foregoing. The works carried out by us earlier confirmed the need to search for coatings and/or methods for its additional processing.
A well known way to protect the surface of titanium is to cover it with gold. This coating increases the corrosion resistance and reduces the ohmic resistance of the fuel cell, which leads to an improvement in its electrical characteristics. However, this technology is
© 2009
M. S. VLASKIN, E. I. SHKOLNIKOV, E. A. KISELEVA, A. A. CHINENOV, V. P. KHARITONOV
costly, mainly due to the use of precious metals.
In this paper, in addition to electrochemical gilding, a method for manufacturing a PB from titanium with its subsequent processing by ion implantation is considered. Alloying the surface of the BP with carbon creates additional corrosion protection and reduces the contact resistance with carbon GDS. This technology promises to reduce the cost of manufacturing PSUs, while maintaining high electrical characteristics.
The paper presents the results of experiments comparing the electrical characteristics of a power supply unit made of "pure" titanium (i.e., without coatings), titanium electrochemically coated with gold, and titanium alloyed with carbon by the ion implantation method.
1. EXPERIMENTAL TECHNIQUE
The current-voltage curve and the FC impedance were chosen as electrical characteristics, with the help of which the above methods of manufacturing a PSU from titanium were compared with each other. The experiments were carried out on a specialized impedancemeter Z-500PX (with the functions of a potentiostat) manufactured by Elins LLC. The FC was loaded with an electronic load built into the impedance in the potentiostatic mode at voltages of 800, 700, 600, and 500 mV. At each voltage, the FC was held for 2000 s to reach a steady state, after which the impedance measurement followed. In each case, after exposure and
when the fuel cell reached the stationary state, 5 hodographs were taken. When measuring the impedance, the amplitude of the perturbing sinusoidal voltage signal was 10 mV, the frequency range was 105–1 Hz. Current-voltage curves were plotted from stationary values.
All experiments were carried out on specially made model test HVFEs (Fig. 1). The test element is a single MEA, sandwiched between two current-collecting plates, which are analogues of the end plates in FC batteries. The overall size of the current collector plates is 28x22 mm, the thickness is 3 mm each. For the convenience of current collection, the plates have special "tails" 4x4 mm. Active surface size 12x18 mm (2.16 cm2). Hydrogen is supplied to the MEA through the anode current collector plate and propagates according to the given flow field on the active surface of this plate. The air feeds the VVTE due to natural convection. The cathode collector plate has 4 channels with a diameter of 2 mm with slots in the area of the active surface. The length of the channel through which the air is distributed is 22 mm. Three-element MEAs are made of Mayop 212, with a platinum catalyst consumption of 0.2 mg/cm2 at the anode and 0.5 mg/cm2 at the cathode.
Test VVTE were assembled from the same components, with the exception of current collector plates. Three pairs of current-collecting plates were made from VT1-0 titanium. The first pair were "pure" ground titanium
Rice. 1. Test fuel cell in a collapsible state. Details from left to right: anode current collector plate, seal, anode GDS, MEA, cathode HDS, seal, cathode current collector plate; bottom - fixing screws and nuts
plates, i.e. without coatings and any additional processing. The second was coated with gold 3 µm thick through a nickel sublayer 2 µm thick by the standard electrochemical method. The third pair was doped with carbon by ion implantation.
The technological process of ion implantation has been known for about 50 years. It is based on the introduction of accelerated ions of a substance into the target material to change the physical and chemical properties of its surface. Ion implantation of titanium BP and end plates was carried out at a specialized stand of CJSC "RIMOS". The stand is an injector capable of creating accelerated ion beams of various substances under conditions of high oil-free vacuum. Titanium plates implanted on this stand have high corrosion resistance and alloying continuity. Titanium plates were subjected to ion-beam treatment at an ion energy of 20 keV, an implantation dose of 1018 cm-2, and a temperature of the processed product of 300 °C ± 10 °C.
The dose of carbon implantation was measured along the depth of the distribution profile of a polished titanium plate by the method of secondary ion mass spectrometry on the CAMECA 1M84B equipment (France). The distribution curve of carbon concentration in titanium is shown in fig. 2. According to the figure, the depth of the carbon surface layer is 200^220 nm, which is sufficient to obtain fundamentally new physical and chemical properties of the BP surface.
1016 _I_I_I_I_I_I_I_I_I_I
0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0
Depth, microns
Rice. 2. Distribution curve of carbon concentration in titanium
2. RESULTS AND DISCUSSION
On fig. Figure 3 shows the volt-ampere curves and the corresponding power density curves for fuel cells with different current-collecting plates. The absolute values of the current and power are related to the MEA active surface area, which is 2.16 cm2. It clearly follows from the figure that both alloying with carbon and electrochemical gilding leads to an improvement in the specific characteristics of fuel cells. It should be noted that the volt-ampere characteristics simultaneously display activation, ohmic, and diffusion losses in a fuel cell. Activation losses are associated with overcoming the energy barrier of electrode reactions, ohmic losses are the sum of the electrical resistances of each of the electrically conductive FC layers and contact resistances between them, and diffusion losses are associated with a lack of supply of reagents to the MEA reaction region. Despite the fact that in various areas of current densities, as a rule, one of the three types of losses listed above prevails, volt-ampere curves and power density curves are not enough to quantification one or another method of processing BP (end plates). In our case, the ohmic losses of FCs are of interest. Activation and diffusion losses are the same for all fuel cells in the first approximation: activation losses due to the use of the same MEA with the same catalyst consumption, diffusion losses due to the same design of test current collector plates.
The hodographs of the impedance obtained in the course of the experiments were used to identify the ohmic losses. The results of this part of the experiments are shown in Figs. 4. As an example, the figures show one of the five hodographs taken in each case after the FC reaches the stationary state.
Impedance spectroscopy makes it possible to quantify the electrical losses of FCs. The papers provide a description this method in relation to VVTE. In accordance with the rules for interpreting hodographs, the ohmic resistance is the real part of the impedance at high frequencies (/ = 105-104 Hz). The value is selected at the point of intersection of the hodograph with the abscissa axis (1m R = 0) in the high frequency region. Also, with the help of hodographs, the capacitance of the double layer on the electrode/electrolyte surface is found. The diameter of the semicircle of the hodograph characterizes the total resistance to the passage of charge through this layer. On fig. 4 impedance hodographs are presented in the range
M. S. VLASKIN, E. I. SHKOLNIKOV, E. A. KISELEVA, A. A. CHINENOV, V. P. KHARITONOV
Rice. 3. Volt-ampere curves (a) and corresponding power density curves (b): - - - uncoated titanium,
W- - titanium + C, -■- - titanium + N1 + Au
0.0 0.2 0.4 0.6 0.8 1.0
1t, From 3.8 3.4 3.0 2.6 2.2 1.8 1.4 1.0 0.6
0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 2.0
0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0
Rice. Fig. 4. TE impedance at constant polarization, mV: a - 800, b - 700 c - 600, d - 500: - uncoated titanium;
Titanium + N1 + Au; o - titanium + C
frequencies of 105-1 Hz, since it is worth noting the rather high diffusion losses of fuel cells (over 2 Ohm-cm2). However, this is not a consequence of the surface treatment of titanium plates, but is associated with the design of the cathode current collector plate and the conditions of natural convection when air is supplied to the MEA.
The table shows the absolute values of ohmic resistances depending on the polarization of the fuel cell and the method of processing its current-collecting plates, as well as their systematic errors. The results indicate that gold plating reduces the total ohmic resistance by a factor of about 1.8 compared to uncoated titanium due to a decrease in contact losses. Doping with carbon ions gives a gain of ∼1.4 times, respectively. The value of the confidence interval indicates the high accuracy of measurements of ohmic resistance values.
Ohmic resistance of a fuel cell (Ohm) with current-collecting plates made of uncoated titanium, titanium electrochemically coated with N1, Au, and titanium doped with C+ ions, depending on the polarization of the fuel cell
Sample TE voltage, mV
Titanium uncoated 0.186 0.172 0.172 0.169
Titanium+Ni, Au 0.1 0.098 0.097 0.093
Titanium+C 0.131 0.13 0.125 0.122
Thus, it has been proven that both gold plating and carbon alloying of titanium BP reduce their contact resistance with carbon HDDs. Coating the wafers with gold turns out to be slightly more advantageous in terms of electrical characteristics than their processing by ion implantation.
All of the above suggests that both one and the other of the considered technologies can be used to process titanium BP.
BIBLIOGRAPHY
1. Middelman E., Kout W, Vogelaar B., Lenssen J., Waal E. de, //J. Power Sources. 2003 Vol. 118. P. 44-46.
2. Dobrovolsky Yu.A., Ukshe A.E., Levchenko A.V., Arkhangelsky I.V., Ionov S.G., Avdeev V.V., Aldoshin S.M. // Journal. Ros. chem. about them. D. I. Mendeleev. 2006. Vol. 1, No. 6. S.83-94.
3. S.-Wang H, Peng J., Lui W.-B., Zhang J.-S. // J. Power Sources. 2006. Vol.162. P.486-491.
4. Davies D.P., Adcock P.L., Turpin M., Rowen S.J., J. Appl. Electrochem. 2000. Vol.30. P.101-105.
5. E. I. Shkolnikov, M. S. Vlaskin, A. S. Ilyukhin, and A. B. Tarasenko, Elektrokhim. energy. 2007. V.7, No. 4 S. 175-182.
6. Shkolnikov E.I., Vlaskin M.S., Iljukhin A.S., Zhuk A.Z., Sheindlin A.E. // J. Power Sources. 2008. Vol.185. P.967-972.
7. Fabian T., Posner J. D., O "Hayre R., Cha S.-W., Eaton J. K., Prinz F. B., Santiago J. G. // J. Power Sources. 2006. Vol. 161. P. 168-182.
8. Ion implantation in semiconductors and other materials: Sat. Art. M.: Mir, 1980.
9. Pleshivtsev N.V., Bazhin A.I. Physics of the impact of ion beams on materials. M.: Vuzovskaya kniga, 1998.
10. Ion implantation. Moscow: Metallurgy, 1985.
11. Pat. 2096856 RF, IPC: H01J027 / 24, H01J003 / 04 / Mashkovtsev BN. Method for producing an ion beam and a device for its implementation.
12. Pat. 2277934 RF, IPC: A61L2/00, A61L2/14 / Kharitonov V.P., Chinenov A.A., Simakov A.I., Samkov A.V. Device for ion-beam processing of medical equipment products.
13. Pat. 2109495 RF, IPC: A61F002/24 / Iosif N.A., Kevorkova R.A.,. Samkov A.V., Simakov A.I., Kharitonov V.P., Chinenov A.A. Artificial heart valve and method for its manufacture.
14. Cooper K.R., Ramani V., Fenton J.M., Kunz H.R. Experimental methods and data analyzes for polymer electrolyte fuel cells, Scribner Associates, Inc., Illinois, 2005. 122 p.
15. National Energy Technology Laboratory. Fuel Cell Hand Book, sixth ed., G&G Services Parsons, Inc. Morgantown, West Virginia, 2002. 352 p.
SOFC electrodes produced at the Institute of Solid State Physics RAS: green - anode and black - cathode. Fuel cells are located on bipolar plates for SOFC batteries
A friend of mine recently visited Antarctica. An amusing trip! she said travel business it is equally developed to bring the traveler to the place and let him enjoy the harsh magnificence of the polar region, without freezing to death. And this is not as easy as it might seem - even taking into account modern technologies: electricity and heat in Antarctica are worth their weight in gold. Judge for yourself, ordinary diesel generators pollute virgin snow, and require delivery a large number fuel, and renewable energy sources are not yet very efficient. For example, at the museum station popular with Antarctic tourists, all energy is generated by the power of wind and sun, but it is cool inside the museum, and four caretakers take a shower only on ships that bring guests to them.
Problems with a constant and uninterrupted power supply are familiar not only to polar explorers, but also to any manufacturers and people living in remote areas.
They can be solved by new ways of storing and generating energy, among which chemical current sources look like the most promising. In these mini-reactors, the energy of chemical transformations directly, without conversion to heat, is converted into electricity. Thus, losses and, accordingly, fuel consumption are sharply reduced.
Different reactions can occur in chemical power sources, and each has its own advantages and disadvantages: some quickly “fail”, others can only work under certain conditions, for example, ultra-high temperatures, or on a strictly defined fuel, such as pure hydrogen. A group of scientists from the Institute of Physics solid body RAS (ISSP RAS) under the direction of Sergei Bredikhin made a bet on the so-called solid oxide fuel cell (SOFC). Scientists are confident that with the right approach, it will be able to replace inefficient generators in the Arctic. Their project was supported under the Federal Target Program "Research and Development for 2014-2020".

Sergey Bredikhin, head of the FTP project "Development of a laboratory scalable technology for the manufacture of planar SOFCs and the concept of creating on their basis power plants for various purposes and structures, including hybrid ones, with the manufacture and testing of a small-scale experimental sample of a power plant with a capacity of 500 - 2000 W"
Without noise and dust, but with full return
Today, the struggle in the energy industry is for a useful energy output: scientists are fighting for every percentage of efficiency. Generators operating on the principle of internal combustion on hydrocarbon fuels - fuel oil, coal, natural gas (the latter type of fuel is the most environmentally friendly) are widely used. Losses during their use are significant: even with maximum optimization, the efficiency of such installations does not exceed 45%. At the same time, during their operation, nitrogen oxides (NOx) are formed, which, when interacting with water in the atmosphere, turn into rather aggressive acids.

SOFC battery under mechanical load
Solid oxide fuel cells (SOFCs) do not have these "side effects". Such installations have an efficiency of more than 50% (and this is only in terms of electricity output, and taking into account the thermal output, the efficiency can reach 85-90%), and they do not emit hazardous compounds into the atmosphere.
“This is a very important technology for the Arctic or Siberia, where the environment and problems with the delivery of fuel are especially important. Because SOFCs consume several times less fuel, Sergey Bredikhin explained. “They have to work non-stop, so they are well suited to work at a polar station, or a northern airfield.”
With a relatively low fuel consumption, such an installation also works without maintenance for up to 3-4 years. “The diesel generator, which is now the most used, requires an oil change every thousand hours. And SOFC works 10-20 thousand hours without maintenance,” Dmitry Agarkov, junior researcher at ISSP, emphasized.
From idea to battery
The operating principle of SOFC is quite simple. They are a "battery" in which several layers of solid oxide fuel cells are assembled. Each element has an anode and a cathode, fuel is supplied to it from the anode side, and air is supplied to it from the cathode side. It is noteworthy that the most suitable for SOFC different types fuels from pure hydrogen to carbon monoxide and various hydrocarbon compounds. As a result of the reactions occurring at the anode and cathode, oxygen and fuel are consumed, and an ion current is created between the electrodes. When a battery is built into an electrical circuit, current begins to flow in that circuit.

Computer simulation of the distribution of currents and temperature fields in a battery of SOFCs 100×100 mm in size.
An unpleasant feature of SOFC operation is the need for high temperatures. For example, a sample collected at the Institute of Solid State Physics, Russian Academy of Sciences, operates at 850°C. To warm up to operating temperature, the generator needs about 10 hours, but then it will work for several years.
The solid oxide cells being developed at the Institute of Solid State Physics RAS will produce up to two kilowatts of electricity, depending on the size of the fuel plate and the number of these plates in the battery. Small mock-ups of 50-watt batteries have already been assembled and tested.
Particular attention should be paid to the plates themselves. One plate consists of seven layers, each of which has its own function. Two layers on the cathode and anode catalyze the reaction and let electrons through, the ceramic layer between them isolates different media (air and fuel), but allows charged oxygen ions to pass through. At the same time, the membrane itself must be strong enough (ceramics of this thickness are very easily damaged), so it itself consists of three layers: the central one gives the necessary physical properties- high ionic conductivity, - and additional layers deposited on both sides give mechanical strength. However, one fuel cell is very thin - no more than 200 microns thick.

SOFC layers
But one fuel cell is not enough - the entire system must be placed in a heat-resistant container that will withstand operation for several years at a temperature of 850 ° C. By the way, as part of the project, to protect metal structural elements, scientists from the Institute of Solid State Physics of the Russian Academy of Sciences use coatings developed in the course of another project.
“When we started this project, we were faced with the fact that we have nothing in our country: no raw materials, no adhesives, no sealants,” Bredikhin said. “We had to do everything. We did simulations, practiced on small fuel cells in the form of pills. We figured out what they should be in terms of composition and configuration, and how they should be located.”
In addition, it must be taken into account that the fuel cell operates in a high temperature environment. This means that it is necessary to ensure tightness, to check that at the target temperature the materials will not react with each other. An important task was to "synchronize" the expansion of all elements, because each material has its own linear coefficient of thermal expansion, and if something is not coordinated, contacts can move away, sealants and adhesives can break. Researchers received a patent for the manufacture of this element.
On the way to implementation
This is probably why the Bredikhin group at the Institute of Solid State Physics has built a whole system of step-by-step preparation of materials first, then plates, and, finally, fuel cells and generators. In addition to this applied wing, there is also a direction dealing with fundamental science.

Within the walls of the Institute of Solid State Physics, rigorous quality control of each batch of fuel cells is carried out.
The main partner in this project is the Krylov State Research Center, which acts as the lead developer of the power plant, including the development of the necessary design documentation and the manufacture of hardware at its pilot plant. Some of the work is done by other organizations. For example, a ceramic membrane that separates the cathode and anode is produced by the Novosibirsk company NEVZ-Ceramics.
By the way, the participation of the shipbuilding center in the project is not accidental. Submarines and underwater drones can become another promising area of SOFC application. For them, too, it is extremely important how long they can be completely offline.
The industrial partner of the project, the Energy Without Borders Foundation, will probably organize the production of small batches of two-kilowatt generators based on Krylovsky scientific center, but scientists hope for a significant expansion of production. According to the developers, the energy received in the SOFC generator is competitive even for domestic use in remote corners of Russia. The cost of a kWh for them is expected to be about 25 rubles, and with the current cost of energy in Yakutia up to 100 rubles per kWh, such a generator looks very attractive. The market has already been prepared, Sergei Bredikhin is sure, the main thing is to have time to prove yourself.
Meanwhile, foreign companies are already introducing generators based on SOFC. The leader in this direction is the American Bloom Energy, which produces 100-kilowatt installations for powerful computer centers of companies such as Google, Bank of America and Walmart.
The practical benefit is clear - huge data centers powered by such generators should be independent of power outages. But in addition, large firms seek to maintain the image of progressive companies that care about environment.
Only here in the United States, large government payments are due for the development of such “green” technologies - up to $ 3,000 for each kilowatt of generated power, which is hundreds of times more than funding for Russian projects.
In Russia, there is another area where the use of SOFC generators looks very promising - this is the cathodic protection of pipelines. First of all, we are talking about gas and oil pipelines that stretch for hundreds of kilometers across the deserted landscape of Siberia. It has been established that when voltage is applied to a metal pipe, it is less susceptible to corrosion. Now cathodic protection stations operate on thermogenerators, which need to be constantly monitored and whose efficiency is only 2%. Their only advantage is their low cost, but if you look at the long term, take into account the cost of fuel (and they are fueled by the contents of the pipe), and this “merit” of them looks unconvincing. With the help of stations based on SOFC generators, it is possible to organize not only an uninterrupted supply of voltage to the pipeline, but also the transmission of electricity for telemetry surveys ... They say that Russia without science is a pipe. It turns out that even this pipe without science and new technologies is a pipe.
The owners of the patent RU 2577860:
The invention relates to a method for protection against oxidation of bipolar plates of fuel cells and current collectors of electrolyzers with a solid polymer electrolyte (SPE), which consists in pretreatment metal substrate, applying an electrically conductive coating of noble metals to the treated metal substrate by magnetron-ion sputtering. The method is characterized by the fact that an electrically conductive coating is applied to the treated substrate in layers, with each layer being fixed by pulsed implantation of oxygen ions or an inert gas. The technical result is to obtain a stable coating with a resource of work, 4 times higher than that obtained by the prototype, and retaining conductive properties. 7 w.p. f-ly, 3 ill., 1 tab., 16 pr.,
Technical field
The invention relates to the field of chemical current sources, and in particular to methods for creating protective coatings for metal current collectors (in the case of electrolyzers) and bipolar plates (in the case of fuel cells - FC) with a solid polymer electrolyte (SPE). During electrolysis, current collectors, usually made of porous titanium, are constantly exposed to aggressive media of oxygen, ozone, hydrogen, which leads to the formation of oxide films on the oxygen current collector (anode), as a result, electrical resistance increases, electrical conductivity and performance decrease. electrolyzer. On the hydrogen collector (cathode) of the current, as a result of hydrogenation of the surface of porous titanium, its corrosion cracking occurs. Working in such harsh conditions with constant humidity, current collectors and bipolar plates need reliable protection against corrosion.
The main requirements for corrosion protective coatings are low electrical contact resistance, high electrical conductivity, good mechanical strength, uniform application over the entire surface area to create electrical contact, low cost of materials and production costs.
For installations with TPE, the most important criterion is the chemical resistance of the coating, the impossibility of using metals that change the degree of oxidation during operation and evaporate, which leads to poisoning of the membrane and catalyst.
Considering all these requirements, Pt, Pd, Ir and their alloys have ideal protective properties.
State of the art
There are currently many known various ways creation of protective coatings - galvanic and thermal recovery, ion implantation, physical vapor deposition (PVD sputtering methods), chemical vapor deposition (CVD sputtering methods).
A method for protecting metal substrates is known from the prior art (U.S. Patent No. 6,887,613 for an invention, published May 3, 2005). The oxide layer, which passivates the surface, was preliminarily removed from the metal surface by chemical etching or mechanical treatment. A polymer coating was applied to the surface of the substrate, mixed with conductive particles of gold, platinum, palladium, nickel, etc. The polymer is selected according to its compatibility with the metal substrate - epoxy resins, silicones, polyphenols, fluorocopolymers, etc. The coating was applied as a thin film using electrophoretic deposition; brush; sprayed in powder form. The coating has good anti-corrosion properties.
The disadvantage of this method is the high electrical resistance of the layer due to the presence of the polymer component.
A protection method is known from the prior art (see US patent US No. 7632592 for the invention, publ. 12/15/2009), which proposes the creation of an anti-corrosion coating on bipolar plates using a kinetic (cold) process of spraying powder of platinum, palladium, rhodium, ruthenium and their alloys. Spraying was carried out with a gun using a compressed gas, such as helium, which is fed into the gun at high pressure. The speed of movement of powder particles is 500-1500 m/s. The accelerated particles remain in a solid and relatively cold state. In the process, their oxidation and melting do not occur, the average layer thickness is 10 nm. The adhesion of particles to the substrate depends on a sufficient amount of energy - with insufficient energy, weak adhesion of particles is observed, at very high energies, deformation of the particles and the substrate occurs, creating high degree local heating.
A method for protecting metal substrates is known from the prior art (see US patent US No. 7700212 for the invention, publ. 20.04.2010). The substrate surface was preliminarily roughened to improve adhesion to the coating material. Two coating layers were applied: 1 - stainless steel, layer thickness from 0.1 μm to 2 μm, 2 - coating layer of gold, platinum, palladium, ruthenium, rhodium and their alloys, no more than 10 nm thick. The layers were applied by thermal spraying, using a gun, from the spray nozzle of which a stream of molten particles was ejected, which formed a chemical bond with the metal surface, it is also possible to apply a coating using the PVD method (physical vapor deposition). The presence of 1 layer reduces the corrosion rate and reduces manufacturing costs, however, its presence also leads to a disadvantage - a passive layer of chromium oxide is formed from stainless steel, which leads to a significant increase in the contact resistance of the anti-corrosion coating.
A protection method is known from the prior art (see US patent No. 7803476 for the invention, publ. 09/28/2010), in which it is proposed to create ultra-thin coatings from the noble metal Pt, Pd, Os, Ru, Ro, Ir and their alloys, thickness coating is from 2 to 10 nm, preferably even a monatomic layer with a thickness of 0.3 to 0.5 nm (thickness equal to the diameter of the coating atom). Previously, a layer of a non-metal having good porosity - coal, graphite mixed with a polymer, or a metal - aluminum, titanium, stainless steel was applied to the bipolar plate. Metal coatings were applied by electron beam sputtering, electrochemical deposition, and magnetron ion sputtering.
The advantages of this method include: elimination of the stage of substrate etching to remove oxides, low contact resistance, minimal cost.
Disadvantages - in the case of a non-metallic layer, the electrical contact resistance increases due to differences in surface energies and other molecular and physical interactions; it is possible to mix the first and second layers, as a result, non-noble metals subject to oxidation may appear on the surface.
A method of protecting a metal substrate is known from the prior art (see US patent No. 7150918 for an invention, published on December 19, 2006), including: processing a metal substrate to remove oxides from its surface, applying an electrically conductive corrosion-resistant metal coating of noble metals, applying an electrically conductive corrosion-resistant polymer coating.
The disadvantage of this method is the high electrical resistance in the presence of a significant amount of binder polymer, in the case of an insufficient amount of binder polymer, electrically conductive soot particles are washed out from the polymer coating.
The prior art method for protecting bipolar plates and current collectors from corrosion is a prototype (see US patent No. 8785080 for the invention, publ. 22.07.2014), including:
Treatment of the substrate in boiling deionized water, or heat treatment at a temperature above 400°C, or soaking in boiling deionized water to form a passive oxide layer with a thickness of 0.5 nm to 30 nm,
Deposition of an electrically conductive metal coating (Pt, Ru, Ir) on a passive oxide layer with a thickness of 0.1 nm to 50 nm. The coating was applied by magnetron-ion sputtering, electron-beam evaporation, or ion deposition.
The presence of a passive oxide layer increases the corrosion resistance of the metal coating, however, and leads to disadvantages - a non-conductive oxide layer sharply worsens the conductive properties of the coatings.
Disclosure of invention
The technical result of the claimed invention is to increase the resistance of the coating to oxidation, increase corrosion resistance and service life and maintain the conductive properties inherent in non-oxidized metal.
The technical result is achieved by the fact that the method of protection against oxidation of bipolar plates of fuel cells and current collectors of electrolyzers with a solid polymer electrolyte (SPE) consists in the fact that the metal substrate is pre-treated, an electrically conductive coating of noble metals is applied to the treated metal substrate by magnetron ion sputtering, in this case, the electrically conductive coating is applied in layers with each layer being fixed by pulsed implantation of oxygen ions or an inert gas.
Preferably, platinum, or palladium, or iridium, or a mixture thereof, is used as noble metals. Pulsed ion implantation is performed with a gradual decrease in ion energy and dose. The total thickness of the coating is from 1 to 500 nm. The successively deposited layers have a thickness from 1 to 50 nm. The inert gas used is argon, or neon, or xenon, or krypton. The energy of implanted ions is from 2 to 15 keV, and the dose of implanted ions is up to 10 15 ions/cm 2 .
Short description drawings
The features and essence of the claimed invention are explained in the following. detailed description, illustrated by drawings and a table showing the following.
In FIG. 1 - distribution of platinum and titanium atoms displaced as a result of argon implantation (calculated by the SRIM program).
In FIG. 2 - a cut of a titanium substrate with sputtered platinum before argon implantation, where
1 - titanium substrate;
2 - a layer of platinum;
3 - pores in the platinum layer.
In FIG. 3 - a cut of a titanium substrate with sputtered platinum after argon implantation, where:
1 - titanium substrate;
4 - intermediate titanium-platinum layer;
5 - platinum coating.
The table shows the characteristics of all examples of implementation of the claimed invention and prototype.
Implementation and examples of the invention
The method of magnetron-ion sputtering is based on a process based on the formation of an annular plasma above the surface of the cathode (target) as a result of the collision of electrons with gas molecules (usually argon). Positive gas ions formed in the discharge, when a negative potential is applied to the substrate, are accelerated in an electric field and knock out atoms (or ions) of the target material, which are deposited on the substrate surface, forming a film on its surface.
The advantages of the magnetron-ion sputtering method are:
High spraying rate of the deposited substance at low operating voltages (400-800 V) and at low pressures of the working gas (5·10 -1 -10 Pa);
Possibility of regulation in a wide range of speed of dispersion and deposition of the sprayed substance;
Low degree of contamination of deposited coatings;
The possibility of simultaneous sputtering of targets from different materials and, as a result, the possibility of obtaining coatings of a complex (multicomponent) composition.
Relative ease of implementation;
Low cost;
Ease of scaling.
At the same time, the resulting coating is characterized by the presence of porosity, has low strength and insufficiently good adhesion to the substrate material due to the low kinetic energy of sputtered atoms (ions), which is approximately 1–20 eV. Such an energy level does not allow the penetration of atoms of the deposited material into the near-surface layers of the substrate material and the creation of an intermediate layer with high affinity for the substrate and coating material, high corrosion resistance, and relatively low resistance even with the formation of an oxide surface film.
Within the framework of the claimed invention, the task of increasing the resistance and maintaining the conductive properties of electrodes and protective coatings of structural materials is solved by exposing the coating and substrate to a stream of accelerated ions that move the coating and substrate material at the atomic level, leading to the interpenetration of the substrate and coating material, resulting in blurring of the interface between the coating and the substrate with the formation of a phase of intermediate composition.
The type of accelerated ions and their energy is selected depending on the coating material, its thickness, and the substrate material in such a way as to cause the movement of coating and substrate atoms and their mixing at the phase boundary with minimal sputtering of the coating material. The selection is made using appropriate calculations.
In FIG. Figure 1 shows the calculated data on the displacement of atoms of a coating consisting of platinum 50 A thick and atoms of a substrate consisting of titanium under the action of argon ions with an energy of 10 keV. Ions with a lower energy at the level of 1-2 keV do not reach the phase boundary and will not provide effective mixing of atoms for such a system at the phase boundary. However, at energies above 10 keV, a significant sputtering of the platinum coating occurs, which negatively affects the service life of the product.
Thus, in the case of a single-layer coating of large thickness and high energy required for the penetration of implanted ions to the phase boundary, the coating atoms are sputtered and precious metals are lost; substrates and coatings and increase the strength of the coating. However, such a small (1–10 nm) coating thickness does not provide a long product life. In order to increase the strength of the coating, its service life and reduce losses during sputtering, pulsed ion implantation is carried out with layer-by-layer (the thickness of each layer is 1-50 nm) coating with a gradual decrease in ion energy and dose. Reducing the energy and dose makes it possible to practically eliminate losses during sputtering, but it makes it possible to ensure the required adhesion of the deposited layers to the substrate, on which the same metal has already been deposited (no phase separation) increases their uniformity. All this also contributes to the increase of the resource. It should be noted that films with a thickness of 1 nm do not provide a significant (required for current collectors) increase in the service life of the product, and the proposed method significantly increases their cost. Films with a thickness of more than 500 nm should also be considered economically unprofitable, since the consumption of platinum group metals increases significantly, and the resource of the product as a whole (cell) begins to be limited by other factors.
When applying multiple layers of coating, treatment with higher energy ions is advisable only after deposition of the first layer with a thickness of 1–10 nm, and when processing subsequent layers with a thickness of up to 10–50 nm, argon ions with an energy of 3–5 keV are sufficient to compact them. The implantation of oxygen ions during the deposition of the first layers of the coating, along with the solution of the above problems, makes it possible to create a corrosion-resistant oxide film on the surface doped with coating atoms.
Example 1 (prototype).
Samples of titanium foil brand VT1-0 area of 1 cm 2 , 0.1 mm thick and porous titanium brand TPP-7 area of 7 cm 2 placed in an oven and kept at a temperature of 450°C for 20 minutes.
The samples are alternately clamped into a frame and placed in a special sample holder of the MIR-1 magnetron-ion sputtering unit with a removable platinum target. The camera is closed. The mechanical pump is switched on and air is evacuated from the chamber to a pressure of ~10 -2 Torr. The chambers block the air evacuation and open the evacuation of the diffusion pump and turn on its heating. After about 30 minutes, the diffusion pump enters the operating mode. The chamber is evacuated through the diffusion pump. After reaching a pressure of 6×10 -5 Torr open the inlet of argon into the chamber. Leakage set the pressure of argon 3×10 -3 Torr. By smoothly increasing the voltage at the cathode, the discharge is ignited, the discharge power is set to 100 W, and the bias voltage is applied. Open the shutter between the target and the holder and start counting the processing time. During processing, the pressure in the chamber and the discharge current are controlled. After 10 minutes of treatment, the discharge is turned off, rotation is turned off, and the argon supply is cut off. After 30 minutes, the pumping out of the chamber is blocked. The heating of the diffusion pump is turned off, and after it has cooled down, the mechanical pump is turned off. The chamber is opened to the atmosphere and the frame with the sample is removed. The thickness of the deposited coating was 40 nm.
The resulting coated materials can be used in electrochemical cells, primarily in electrolyzers with a solid polymer electrolyte, as cathode and anode materials (current collectors, bipolar plates). Anode materials cause the most problems (intense oxidation); therefore, life tests were carried out when they were used as anodes (that is, at a positive potential).
To the obtained sample of titanium foil by the method spot welding a current lead is welded and placed as a test electrode in a three-electrode cell. Pt foil with an area of 10 cm 2 is used as a counter electrode, and a standard silver chloride electrode connected to the cell through a capillary is used as a reference electrode. The electrolyte used is a solution of 1M H 2 SO 4 in water. Measurements are carried out using an AZRIVK 10-0.05A-6 V device (manufactured by LLC "Buster", St. Petersburg) in a galvanostatic mode, i.e. a positive direct current potential is applied to the electrode under study, which is necessary to achieve a current value of 50 mA. The test consists of measuring the change in potential required to reach a given current over time. If the potential exceeds the value of 3.2 V, the electrode resource is considered exhausted. The resulting sample has a resource of 2 hours 15 minutes.
Examples 2-16 of the implementation of the claimed invention.
Samples of titanium foil brand VT1-0 with an area of 1 cm 2 , 0.1 mm thick and porous titanium brand TPP-7 area of 7 cm 2 boiled in isopropyl alcohol for 15 minutes. Then the alcohol is drained off and the samples are boiled 2 times for 15 minutes in deionized water with water changes between boils. The samples are heated in a solution of 15% hydrochloric acid to 70°C and maintained at this temperature for 20 minutes. The acid is then drained off and the samples are boiled 3 times for 20 minutes in deionized water with water changes between boils.
The samples are alternately placed in a MIR-1 magnetron-ion sputtering unit with a platinum target and a platinum coating is applied. The magnetron current is 0.1 A, the magnetron voltage is 420 V, the gas is argon with a residual pressure of 0.86 Pa. For 15 minutes of deposition, a coating with a thickness of 60 nm is obtained. The resulting coating is exposed to the flow of argon ions by the method of plasma pulsed ion implantation.
Implantation is carried out in a stream of argon ions with a maximum ion energy of 10 keV, an average energy of 5 keV. The dose during exposure was 2*10 14 ions /cm 2 . The sectional view of the coating after implantation is shown in Fig. 3.
The resulting sample is tested in a three-electrode cell, the process is similar to that shown in example 1. The resulting sample has a resource of 4 hours. For comparison, the data on the resource of titanium foil with the initial sputtered platinum film (60 nm) without argon implantation is 1 hour.
Examples 3-7.
The process is similar to that in example 2, but the implantation dose, ion energy and coating thickness are varied. The implantation dose, ion energy, coating thickness, as well as the service life of the obtained samples are shown in Table 1.
The process is similar to that shown in example 2 and differs in that samples with a deposited layer thickness of up to 15 nm are processed in a krypton flow with a maximum ion energy of 10 keV and a dose of 6*10 14 ions/cm 2 . The resulting sample has a resource of 1 hour 20 minutes. According to electron microscopy, the thickness of the platinum layer was reduced to a value of 0–4 nm, but a titanium layer with platinum atoms embedded in it was formed.
The process is similar to that shown in example 2 and differs in that samples with a deposited layer thickness of 10 nm are processed in an argon ion flow with a maximum ion energy of 10 keV and a dose of 6*10 14 ions/cm 2 . After deposition of the second layer with a thickness of 10 nm, processing is carried out in a flow of argon ions with an energy of 5 keV and a dose of 2*10 14 ion/cm 2, and then the deposition is repeated 4 times with a thickness of a new layer of 15 nm, and each subsequent layer is processed in a flow of ions argon with an ion energy of 3 keV and a dose of 8*10 13 ion/cm 2 . The resulting sample has a resource of 8 hours 55 minutes.
Example 10
The process is similar to that shown in example 2 and differs in that samples with a deposited layer thickness of 10 nm are treated in an oxygen ion flow with a maximum ion energy of 10 keV and a dose of 2*10 14 ion/cm 2 . After deposition of the second layer 10 nm thick, treatment is carried out in a flow of argon ions with an energy of 5 keV and a dose of 1*10 14 ion/cm 2 , and then the deposition is repeated 4 times with a new layer thickness of 15 nm, with each subsequent layer being treated in a flow of argon ions with an ion energy of 5 keV and a dose of 8 * 10 13 ion / cm 2 (so that there is no sputtering!). The resulting sample has a resource of 9 hours 10 minutes.
Example 11.
The process is similar to that shown in example 2 and differs in that the samples are placed in the MIR-1 magnetron-ion sputtering unit with an iridium target and an iridium coating is applied. The magnetron current is 0.1 A, the magnetron voltage is 440 V, the gas is argon with a residual pressure of 0.71 Pa. The deposition rate ensures the formation of a coating with a thickness of 60 nm in 18 minutes. The resulting coating is exposed to the flow of argon ions by the method of plasma pulsed ion implantation.
Samples with a first deposited layer thickness of 10 nm are treated in an argon ion flow with a maximum ion energy of 10 keV and a dose of 2*10 14 ion/cm 2 . After deposition of the second layer with a thickness of 10 nm, treatment is carried out in a stream of argon ions with an energy of 5-10 keV and a dose of 2*10 14 ion/cm 2, and then the deposition is repeated 4 times with a new layer thickness of 15 nm, each subsequent layer is treated in a stream argon ions with an ion energy of 3 keV and a dose of 8*10 13 ion/cm 2 . The resulting sample has a resource of 8 hours 35 minutes.
Example 12.
The process is similar to that shown in example 2 and differs in that the samples are placed in a MIR-1 magnetron-ion sputtering installation with a target made of an alloy of platinum with iridium (Pli-30 alloy according to GOST 13498-79), a coating is applied consisting of platinum and iridium. The magnetron current is 0.1 A, the magnetron voltage is 440 V, the gas is argon with a residual pressure of 0.69 Pa. The deposition rate ensures the formation of a coating with a thickness of 60 nm in 18 minutes. The resulting coating is exposed to the flow of argon ions by the method of plasma pulsed ion implantation.
Samples with a deposited layer thickness of 10 nm are treated in an argon ion flow with a maximum ion energy of 10 keV and a dose of 2*10 14 ion/cm 2 , and then the deposition is repeated 5 times with a new layer thickness of 10 nm. After applying the second layer, treatment is carried out in a flow of argon ions with an energy of 5-10 keV and a dose of 2*10 14 ion/cm 2, and each subsequent layer is treated in a flow of argon ions with an ion energy of 3 keV and a dose of 8*10 13 ion/cm 2. The resulting sample has a resource of 8 hours 45 minutes.
Example 13
The process is similar to that shown in example 2 and differs in that the samples are placed in the MIR-1 magnetron-ion sputtering unit with a palladium target and a palladium coating is applied. The magnetron current is 0.1 A, the magnetron voltage is 420 V, the gas is argon with a residual pressure of 0.92 Pa. For 17 minutes of deposition, a coating with a thickness of 60 nm is obtained. Samples with a deposited first layer thickness of 10 nm are treated in an argon ion flow with a maximum ion energy of 10 keV and a dose of 2*10 14 ion/cm 2 . After deposition of the second layer with a thickness of 10 nm, treatment is carried out in a stream of argon ions with an energy of 5-10 keV and a dose of 2*10 14 ion/cm 2, and then the deposition is repeated 4 times with a new layer thickness of 15 nm, each subsequent layer is treated in a stream argon ions with an ion energy of 3 keV and a dose of 8*10 13 ion/cm 2 . The resulting sample has a resource of 3 hours 20 minutes.
Example 14
The process is similar to that in example 2 and differs in that the samples are placed in a MIR-1 magnetron-ion sputtering installation with a target consisting of platinum, including 30% carbon, and a coating consisting of platinum and carbon is applied. The magnetron current is 0.1 A, the magnetron voltage is 420 V, the gas is argon with a residual pressure of 0.92 Pa. For 20 minutes of deposition, a coating with a thickness of 80 nm is obtained. Samples with a deposited layer thickness of 60 nm are treated in an argon ion flow with a maximum ion energy of 10 keV and a dose of 2*10 14 ion/cm 2 , and then sputtering is repeated 5 times with a new layer thickness of 10 nm. After applying the second layer, treatment is carried out in a flow of argon ions with an energy of 5-10 keV and a dose of 2*10 14 ion/cm 2, and each subsequent layer is treated in a flow of argon ions with an ion energy of 3 keV and a dose of 8*10 13 ion/cm 2. The resulting sample has a resource of 4 hours 30 minutes.
Example 15
The process is similar to that given in example 9 and differs in that 13 layers are deposited, the thickness of the first and second is 30 nm each, the subsequent ones are 50 nm each, the ion energy is successively reduced from 15 to 3 keV, the implantation dose is from 5 10 14 to 8 10 13 ion/cm2. The resulting sample has a resource of 8 hours 50 minutes.
Example 16
The process is similar to that shown in example 9 and differs in that the thickness of the first layer is 30 nm, the next six layers are 50 nm each, the implantation dose is from 2·10 14 to 8·10 13 ion/cm 2 . The resulting sample has a resource of 9 hours 05 minutes.
Thus, the claimed method of protecting bipolar FC plates and current collectors of TPE electrolyzers from oxidation makes it possible to obtain a stable coating with a service life 4 times higher than that obtained according to the prototype, and retaining conductive properties.


1. A method for protecting bipolar plates of fuel cells and current collectors of electrolyzers with a solid polymer electrolyte (SPE) from oxidation, which consists in pre-treatment of a metal substrate, applying an electrically conductive coating of noble metals to the treated metal substrate by magnetron ion sputtering, characterized in that it is applied to the treated substrate is an electrically conductive coating layer by layer with fixing of each layer by pulsed implantation of oxygen ions or an inert gas.
2. The method of protection according to claim 1, characterized in that platinum, or palladium, or iridium, or a mixture thereof is used as noble metals.
3. The method of protection according to claim 1, characterized in that pulsed ion implantation is performed with a gradual decrease in ion energy and dose.
4. The method of protection according to claim 1, characterized in that the total thickness of the coating is from 1 to 500 nm.
5. The method of protection according to claim 1, characterized in that the successively deposited layers have a thickness of 1 to 50 nm.
6. The protection method according to claim 1, characterized in that argon, or neon, or xenon, or krypton is used as an inert gas.
7. The method of protection according to claim 1, characterized in that the energy of the implanted ions is from 2 to 15 keV.
8. The method of protection according to claim 1, characterized in that the dose of implanted ions is up to 10 15 ions/cm 2 .
Similar patents:
The invention relates to the field of electrical engineering, namely to a battery of tubular solid oxide fuel cells (SOFC), which includes at least two nodes of tubular solid oxide fuel cells, at least one common current collector and a holder for holding a section of fuel cell assemblies and a common current collector in connecting with them with an exact fit, while the coefficient of thermal expansion of the holder is less than or equal to the coefficient of thermal expansion of the fuel cell assemblies.
The invention relates to polymer membranes for low or high temperature polymer fuel cells. A proton-conducting polymeric membrane based on a polyelectrolyte complex consisting of: a) a nitrogen-containing polymer such as poly-(4-vinylpyridine) and its derivatives obtained by alkylation, poly-(2-vinylpyridine) and its derivatives obtained by alkylation, polyethyleneimine, poly(2-dimethylamino)ethylmethacrylate)methyl chloride, poly(2-dimethylamino)ethylmethacrylate)methyl bromide, poly(diallyldimethylammonium) chloride, poly(diallyldimethylammonium) bromide, b) Nafion or another Nafion-like polymer selected from the group, including Flemion, Aciplex, Dowmembrane, Neosepta and ion exchange resins containing carboxyl and sulfonic groups; c) a liquid mixture comprising a solvent selected from the group consisting of methanol, ethyl alcohol, n-propyl alcohol, isopropyl alcohol, n-butyl alcohol, isobutyl alcohol, tert-butyl alcohol, formamides, acetamides, dimethyl sulfoxide, N-methylpyrrollidone, and also distilled water and mixtures thereof; in which the molar ratio of nitrogen-containing polymer to Nafion or Nafion-like polymer is in the range of 10-0.001.
The invention relates to the field of electrical engineering, namely to obtaining an oxide film of electrolyte with a thickness comparable to the pore size of the electrode material, in a simpler and more technologically advanced, and also more economical way than ion-plasma.
The invention provides a fuel cell gas diffusion medium that has low in-plane air permeability and good drainage property and is capable of high fuel cell performance over a wide temperature range from low to high temperatures.
The invention relates to the field of electrical engineering, namely to a method for manufacturing a catalytic electrode of a membrane-electrode unit, mainly for hydrogen and methanol fuel cells.
The invention relates to the field of electrical engineering and can be used in fuel cells. The bipolar fuel cell plate includes the plate, a fluid flow space formed on both sides of the plate, a fluid guide grid installed in the fluid flow space. The plate is formed with an inlet channel connected to the fluid flow space and an outlet channel connected to the fluid flow space. The bipolar plate is made using a certain mold and appropriate processing. The result is a more uniform flow distribution and a reduction in resistance to fuel and air flows to the fuel electrode and air electrode of the fuel cell, respectively. In addition, the reaction area with the membrane electrode assembly and the diffusion zone can be increased, and the fabrication can be simplified and facilitated, 6N. and 14 z.p. f-ly, 16 ill.
Technical field
The invention relates to a fuel cell and, in particular, to a bipolar fuel cell plate and a method for manufacturing such a plate capable of imparting uniform flow distribution, reducing resistance to fuel and air flows flowing into the fuel electrode and air electrode of the fuel cell, respectively, and simplifying its manufacture .
State of the art
The fuel cell generates generally environmentally friendly energy, and it was designed to replace traditional fossil fuel energy. As shown in FIG. 1, the fuel cell includes a stack 100 to be combined with at least one single cell 101 in which an electrochemical reaction takes place; a fuel supply line 200 connected to the stack 100 so as to supply fuel; a supply air duct 300 connected to the stack 100 so as to supply air; and exhaust conduits 400, 500 for discharging by-products of the ongoing fuel and air reaction, respectively. The unit cell 101 includes a fuel electrode (anode) (not shown) to which fuel is supplied; and an air electrode (cathode) (not shown) to which air is supplied.
First, fuel and air are supplied to the fuel electrode and the air electrode of the stack 100 via the fuel supply line 200 and the air supply line 300, respectively. The fuel supplied to the fuel electrode is ionized into positive ions and electrons (e-) through an electrochemical oxidation reaction at the fuel electrode, the ionized positive ions move through the electrolyte to the air electrode, and the electrons move to the fuel electrode. The positive ions transferred to the air electrode enter into an electrochemical reduction reaction with the air supplied to the air electrode and generate by-products such as heat of reaction and water, etc. In this process, the movement of electrons generates electricity. The fuel after the reaction at the fuel electrode, as well as the water and additional by-products generated at the air electrode, are discharged through the exhaust conduits 400, 500, respectively.
Fuel cells can be classified into different types according to the electrolyte and fuel used in them, etc.
Meanwhile, as shown in FIG. 2, the single element 101 constituting the stack 100 includes two bipolar plates 10 having an open passage 11 through which air or fuel flows; and a membrane-electrode assembly (MEA, from the English "membrane electrode assembly" or MEA) 20, placed between these two bipolar plates 10 so as to have a certain thickness and area. Two bipolar plates 10 and MEU 20 placed between them are combined with each other by means of additional means 30, 31 of the association. The channel formed by the channel 11 of the bipolar plate 10 and the side of the MED 20 constitutes the fuel electrode, and when fuel flows through this channel of the fuel electrode, an oxidation reaction occurs. In addition, the channel formed by the channel 11 of the other bipolar plate 10 and the other side of the MED 20 constitutes an air electrode, and when air flows through this channel of the air electrode, a reduction reaction occurs.
The shape of the bipolar plate 10, in particular the shape of the channel 11, affects the contact resistance provided by the flow of fuel and air and the distribution of flows and the like, and the contact resistance and the distribution of flows affect the power output (energy efficiency). In addition, the bipolar plates 10 have a certain shape suitable for facilitating the process and mass production.
As shown in FIG. 3, through holes 13, 14, 15, 16, respectively, are formed in the conventional bipolar plate at each edge of the plate 12 having a certain thickness and a rectangular shape.
In addition, multiple channels 11 are formed on the side of the plate 12 so as to connect the through hole 13 to the diagonally located through hole 16. These channels 11 are zigzag-shaped. As shown in figure 4, in the cross section of the channel 11, this channel 11 has a certain width and thickness and one open side. On the other side of the plate 12, multiple channels 11 are formed so as to connect two diagonally arranged through holes 14, 16, these channels 11 having the same shape as the channels formed on the opposite side.
The following describes the operation of a traditional bipolar plate. First, fuel and air flow into the through holes 13, 14, respectively, and the fuel and air passing through the through holes 13, 14 flow into the channels 11. The fuel or air in the channels 11 flows in a zigzag pattern along the channels 11 and is discharged to the outside through the through holes 15, 16. In this process, in the MED 20 (shown in FIG. 2) in which fuel flows, an oxidation reaction occurs, and simultaneously a reduction reaction occurs in the MED in which air flows.
However, in the case of a conventional bipolar plate, since the channels 11 are formed in a zigzag manner, the flow can only be uniformly distributed to some extent. Moreover, since the channels through which the fuel and air flow are complex and long, the resistance to flow increases and therefore the pressure loss to create the flow of fuel and air increases. In addition, since the manufacturing process is complex and cumbersome, the production cost is high.
Technical essence of the present invention
In order to solve the problems described above, it is an object of the present invention to provide a bipolar fuel cell plate and a method for manufacturing such a plate capable of imparting uniform flow distribution, reducing resistance to fuel and air flows flowing into the fuel electrode and air electrode of the fuel cell, respectively, and simplify its production.
In order to achieve the above objects, the bipolar fuel cell plate includes a plate having a certain thickness and area; a fluid flow space formed on both sides of this plate so as to have a certain width, length and depth; a fluid guide grid installed in the fluid flow space so as to have a certain shape; an inlet port formed on the plate to be connected to the fluid flow space and receive the fluid; and an outlet port formed on the plate so as to be connected to the fluid flow space and discharge the fluid.
In addition, the method for manufacturing a bipolar fuel cell plate includes manufacturing a mold for processing the plate, on which a fluid flow space having a certain area and depth is formed on both sides, and an internal channel is formed by means of a support grid protruding in the shape of a grid. from the fluid flow space; forming a plate with this mold; processing the plate with the implementation of the inlet so as to allow the inflow of fluid flow into the fluid flow space having a support grid; and processing the plate to form an outlet so as to allow flow to flow out of the fluid flow space.
In addition, the bipolar fuel cell plate includes a plate having a certain thickness and area; a channel area having lattice protrusions next to multiple lattice grooves formed along a certain area of both sides of the plate; an inlet channel formed on the side of the plate so as to be connected with the lattice slots in the channel area and receive the fluid; and an outlet channel formed on the side of the plate so as to discharge the fluid passing through the lattice slots of the channel area.
In addition, the method for manufacturing a bipolar fuel cell plate includes manufacturing a plate having a certain thickness and area; performance machining for forming lattice slots next to the lattice protrusions formed on both sides of the plate; and processing the plate to form an inlet and an outlet so that they are connected to the lattice slots.
In addition, the bipolar fuel cell plate includes a plate having a certain thickness and area, in which on both sides in the middle, multiple channels composed of multiple ups and downs are formed by pressing to have a certain width and length; and a sealing member respectively attached to the contour of both sides of the plate so as to form internal channels, together with the plate channels, an inlet channel and an outlet channel through which fluid flows into and out of these channels.
In addition, the method for manufacturing a bipolar fuel cell plate includes cutting the plate so that it has a certain size; pressing both sides of the cut plate so as to form multiple channels through which fluid flows; and combining the sealing element with the contour of the press-worked plate.
Brief description of the drawings
The accompanying drawings, which are included to provide a better understanding of the invention, form part of and form part of this specification, illustrate embodiments of the invention and, together with the description, serve to explain the principles of the invention.
On these drawings:
Fig. 1 illustrates a conventional fuel cell system;
Fig. 2 is an exploded perspective view illustrating part of a conventional fuel cell package;
Fig. 3 is a plan view illustrating a bipolar plate of a conventional fuel cell;
Fig. 4 is a sectional view along line A-B in Fig. 3;
Fig. 5 is a plan view illustrating a first embodiment of a bipolar fuel cell plate according to the present invention;
Fig. 6 is an exploded perspective view illustrating a portion of a bipolar fuel cell plate according to a first embodiment of the present invention;
7 is a flowchart illustrating a first embodiment of a method for manufacturing a bipolar fuel cell plate according to the present invention;
Fig. 8 is an exploded perspective view illustrating a bipolar plate stack of a fuel cell according to a first embodiment of the present invention;
Fig. 9 is a plan view illustrating an operating state of the bipolar fuel cell plate according to the first embodiment of the present invention;
10 and 11 are top and front sectional views illustrating a second embodiment of a bipolar fuel cell plate in accordance with the present invention;
12 is a flowchart illustrating a second embodiment of a method for manufacturing a bipolar fuel cell plate according to the present invention;
Fig. 13 is a plan view illustrating an operating state of the bipolar fuel cell plate according to the second embodiment of the present invention;
14 and 15 are top and front sectional views illustrating a third embodiment of a bipolar fuel cell plate according to the present invention; and
16 is a flowchart illustrating a third embodiment of a method for manufacturing a bipolar fuel cell plate according to the present invention.
First, a first embodiment of the bipolar fuel cell plate according to the present invention will be described.
Fig. 5 is a plan view illustrating a first embodiment of a bipolar fuel cell plate according to the present invention, and Fig. 6 is an exploded perspective view illustrating a part of a bipolar fuel cell plate according to a first embodiment of the present invention. .
As shown in FIGS. 5 and 6, a first embodiment of a bipolar fuel cell plate according to the present invention includes a plate 40 having a certain thickness and area; a fluid flow space 41 formed on both sides of the plate 40 so as to have a certain width, length, and depth; a fluid direction grid 42 installed in the fluid flow space 41 so as to have a certain shape; an inlet port 43 formed on the plate 40 connected to the fluid flow space 41 for introducing the fluid; and an outlet port 44 formed on the plate 40 connected to the fluid flow space 41 to discharge the fluid.
The plate 40 has a rectangular shape and a certain thickness, a fluid flow space 41 is formed respectively on both sides of the rectangular plate 40, and it has a rectangular shape and a certain depth. The plate 40 is made of a stainless steel material. Plate 40 and fluid flow space 41 may have shapes other than rectangular.
The fluid direction mesh 42 has a rectangular shape smaller than the fluid flow space 41 so that it can be inserted into the fluid flow space 41 of the plate 40, and it has a thickness not greater than the depth of the fluid flow space 41 .
The inlet port 43 is formed as at least one through hole and is formed on one side of the plate 40. The outlet port 43 is made as at least one through hole and is formed on the opposite side from the inlet port 43 so as to be diagonal with respect to this inlet 43.
7 is a flowchart illustrating a first embodiment of a method for manufacturing a bipolar fuel cell plate in accordance with the present invention.
As shown in FIG. 7, in the first embodiment of the method for manufacturing a bipolar fuel cell plate according to the present invention, a mold for processing the plate is formed, on which a fluid flow space having a certain area and depth is formed on both sides, and formed a grid protruding into the flow space of the fluid medium. After that, the plate is processed using this mold. At the same time, a rectangular fluid flow space having a certain depth is formed in the plate on both sides of the rectangular plate having a certain depth, and a grid is formed in the fluid flow space so as to form a channel. This mesh can be formed into various shapes.
Next, the plate is processed to form an inlet so as to allow fluid flow to flow into the meshed fluid flow space, and processed to form an outlet so as to allow flow to flow out of the fluid flow space. The inlet channel and the outlet channel, respectively, are made in the form of at least one through hole or open groove.
First, the bipolar fuel cell plates are stacked. In more detail, as shown in Fig. 8, MEAs (M) are placed between the bipolar plates (BP) and they are combined with each other by means of a combination (not shown). In this case, the fluid flow space 41 formed on the side of the bipolar plate (BP), the fluid direction grid 42 formed in the fluid flow space 41, and the side of the MED (M) form a path (channel) through which the fuel flows. The other side of the MED (M), the fluid flow space 41 formed on the side of the other bipolar plate (BP) facing the first bipolar plate (BP), and the fluid direction grid 42 formed in the fluid flow space 41 are formed path (channel) through which air flows.
With this structure, when fuel is supplied to the bipolar plate (BP) inlet 43 as shown in FIG. 9, the fuel in the inlet 43 flows into the fluid flow space 41 . Further, the fuel in the fluid flow space 41 is spread (distributed) throughout the fluid flow space 41 by the fluid guide grid 42 placed in the fluid flow space 41, and then the fuel is discharged to the outside through the outlet port 44.
In this process, the fluid guiding grid 42 in the fluid flow space 41 performs not only a guiding function by evenly spreading the fuel in the fluid flow space 41, but also a "diffusion" function (diffusion function) by properly controlling the flow density. In this case, the distribution and pressure can be adjusted by the size of the "cells" of the mesh 42 of the direction of the fluid. Meanwhile, due to the formation of the fluid direction grid 42 in the form of a grid, the contact area with the MED (M) in contact with the bipolar plate (BP) is relatively reduced, and, accordingly, the effective contact area of the fuel and MED (M) is increased.
In addition to this, the air flows through the same process as described above.
In the case of the method for manufacturing a bipolar fuel cell plate according to the first embodiment of the present invention, by manufacturing the plate with a mold, it can be easily mass-produced. In more detail, by manufacturing the support grid plate and making the inlet and outlet, the bipolar plate can be simply and easily manufactured.
10 and 11 are top and front sectional views illustrating a second embodiment of a bipolar fuel cell plate in accordance with the present invention.
As shown in FIGS. 10 and 11, the bipolar fuel cell plate according to the second embodiment of the invention includes a plate 50 having a certain thickness and area; a channel area 53 having lattice protrusions 52 adjacent to multiple lattice grooves 51 formed along a certain area of both sides of the plate 50; an inlet 54 formed on one side of the plate 50 so as to be connected to the lattice slots 51 of the fluid passage area 53; and an outlet channel 55 formed on this side of the plate 50 so as to discharge the fluid passing through the lattice slots 51 of the channel area 53.
The plate 50 has a rectangular shape and a certain thickness. The channel region 53 is respectively formed on both sides of the plate 50 so as to have a rectangular shape. The plate 50 and the channel region 53 may be formed into various shapes other than a rectangular shape.
The lattice protrusions 52 are formed in the shape of a rectangular cone, and each lattice groove 51 is formed between these lattice protrusions 52 in the form of a rectangular cone. The lattice protrusion 52 may be formed to have the shape of a triangular cone.
The lattice protrusions 52 are arranged in a regular manner (at regular intervals). In one modification, the lattice protrusions 52 may be placed in an irregular manner.
The inlet port 54 and the outlet port 55, respectively, are formed on one side of the plate 50 having open form, with defined width and depth. In addition, the inlet port 54 and the outlet port 55 may be respectively formed as at least one through hole.
The bipolar fuel cell plate according to the second embodiment of the present invention is made of stainless steel.
12 is a flowchart illustrating a second embodiment of a method for manufacturing a bipolar fuel cell plate according to the present invention.
As shown in FIG. 12, in the method for manufacturing a bipolar fuel cell plate according to the second embodiment of the present invention, the first step is to manufacture a plate having a certain thickness and area. A second step is then performed in the form of machining to form the lattice slots next to the lattice protrusions on both sides of the plate. This second step includes the sub-steps of notching both sides of the plate to form lattice projections; and grinding both notched sides of the plate. The lattice protrusions formed by the notch are in the shape of a rectangular cone, but they can be formed in shapes other than the rectangular cone. By knurling, lattice slots are formed among the lattice protrusions, wherein the lattice slots form channels through which fluid flows. By performing grinding, it is possible to remove the burrs generated by the notching and to process the sharp ends (tops) of the lattice protrusions so that they are blunt.
Finally, the third step is to machine the plate to form an inlet and an outlet so that they are connected to the lattice slots.
The bipolar fuel cell plates are assembled into a package. In this case, the channel area 53 formed on one side of the bipolar plate (BP) and the side of the MEU (M) forms a path (channel) through which the fuel flows. The other side of the MED (M) and the side of the other bipolar plate (BP) facing the first bipolar plate (BP) form a path (channel) through which air flows.
With this design, when fuel is supplied to the inlet passage 54 of the bipolar plate (BP) as shown in FIG. 53 channels, and then this fuel is discharged to the outside through the outlet channel 55.
In this process, due to the small and uniform shape of such a grid formed by the lattice slots 51 formed by the lattice protrusions 52 in the channel area 53, the fluid can not only be uniformly distributed but also dissipated. At the same time, due to the lattice protrusions 52 formed in the region 53 of the channel, the contact area of the bipolar plate (BP) and the MEA (M) is relatively reduced, and the effective contact area of the fuel and the MEA (M) is increased.
In addition to this, the air flows through the same process as described above.
In the case of the method for manufacturing a bipolar fuel cell plate according to the second embodiment of the present invention, by machining a rectangular plate having a certain thickness on both sides to form an inlet and an outlet with a roll, etc., the manufacture is simple and fast.
14 and 15 are top and front sectional views illustrating a third embodiment of a bipolar fuel cell plate in accordance with the present invention.
As shown in FIGS. 14 and 15, the bipolar fuel cell plate according to the third embodiment of the present invention includes a plate 60 having a certain thickness and area, in which, on both sides in the middle, multiple channels 61 composed of numerous ascents and descents, so that they have a certain width and length; and a sealing member 65 respectively attached to the contour of both sides of the plate 60 so as to form channels 62a, 62b, 62c together with the channels 61 of the plate 60, an inlet 63 and an outlet 64 through which fluid flows in and out.
The plate 60 is made in the form of a rectangular metal plate, and channels 61 are formed in a certain inner area of this rectangular metal plate. When the plate 60 is pressed, the channels 61 are respectively formed on both sides of the plate 60, and the channels 61 have the same depth.
The sealing element 65 has a rectangular shape and a certain width, and it has the same thickness as the height of the risers of the channel 61, and has the same size as the plate 60. The height of the rises of the channel 61 is approximately 2.5 mm.
An inlet 63 through which fluid flows is formed on one side of the sealing member 65, and an outlet 64 is formed to be opposite the inlet 63.
The inner channel formed by the sealing member 65 includes an inlet buffer channel 62a for distributing fluid through the channels 61 of the plate 60; an outlet buffer channel 62b for allowing the fluid passing through the channels 61 of the plate 60 to flow into the outlet channel 64; and a connecting channel 62c for connecting the inlet buffer channel 62a and the outlet buffer channel 62b.
16 is a flowchart illustrating a third embodiment of a method for manufacturing a bipolar fuel cell plate according to the present invention.
As shown in FIG. 16, in the manufacturing method of the bipolar fuel cell plate according to the third embodiment of the present invention, the first step is to obtain a plate 60 by cutting a metal plate having a certain thickness and area according to a certain size, and the second step is to press-process the plate. 60 so as to form multiple channels 61 on both sides of the plate 60. The metal plate 60 has a rectangular shape.
The channels 61 of the plate 60 are made straight, and they have a certain length, and the height of the rises of the channels 61 is the same. The channel 61 of the plate 60 may have various cross-sectional shapes, such as a wave shape or a rectangular shape.
The third step is to combine the sealing member 65 with the contour of the press-formed plate 60. The sealing member 65 is formed into a rectangular spacer having a certain width and thickness, and this sealing member 65 is combined with the contour of the plate 60 so as to surround the inner region of the plate 60, and hence form channels 62a, 62b, 62c. An inlet 63 and an outlet 64 are formed on the sealing member 65. The inlet 63 and the outlet 64 may be formed by cutting a portion of the sealing member 65.
As described above in the first embodiment of the present invention, the fuel cell package is assembled. At the same time, by lifting the straight channel 61 formed on the side of the bipolar plate (BP) and the side of the MEU (M), a path (channel) is formed along which the fuel flows. The other side of the MED (M) and the slopes of straight channels 61 formed on the side of the other bipolar plate (BP) facing the first bipolar plate (BP) form a path (channel) through which air flows.
With this design, when fuel is supplied to the bipolar plate (BP) inlet port 63, the fuel in the inlet port 63 flows along this path, namely through the inlet buffer port 62a, the connecting port 62c, the port 61 and the outlet buffer port 62b. Thereafter, the fuel is discharged to the outside through the outlet port 64. In addition, air flows through the same process as described above.
In addition, in the present invention, by manufacturing the metal plate by press processing, the manufacturing is simple and fast. In addition, by reducing the thickness of the bipolar plate, the size and weight of the package can be reduced.
Industrial Applicability
As described above, in the case of the bipolar fuel cell plate and the manufacturing method thereof according to the present invention, by making the fuel and air flows respectively flowing into the fuel electrode and the air electrode of the fuel cell uniform, increasing the effective area of reaction with the MEA, and increasing the diffusion zone power output (energy output) can be increased. By reducing the resistance to the flow of fuel and air, the pressure loss generating the flow of fuel and air can be reduced, i. e. pumping force. In addition, by simplifying and facilitating manufacturing, production costs can be greatly reduced, and therefore mass production is possible.
1. Bipolar fuel cell plate, containing a plate having a certain thickness and area; a fluid flow space formed on both sides of the plate, the fluid flow space being configured to have a certain width, length, and depth; a fluid guide grid installed in the fluid flow space, the fluid guide grid having a certain shape; an inlet port formed on the plate connected to the fluid flow space for introducing the fluid; and an outlet port formed on the plate connected to the fluid flow space to discharge the fluid.
2. The bipolar plate according to claim 1, wherein the fluid flow space is formed to be rectangular in shape, and the fluid guide grid is rectangular in shape not larger than the size of the fluid flow space.
3. The bipolar plate of claim 1, wherein the fluid guide grid has a thickness no greater than the depth of the fluid flow space.
4. The bipolar plate according to claim 1, wherein the inlet port and the outlet port are respectively formed as at least one through hole and are formed on the side of the plate.
5. The bipolar plate of claim 1, wherein the inlet and outlet are diagonal to each other.
6. The bipolar plate according to claim 1, the plate being made of a stainless steel material.
7. A method for manufacturing a bipolar fuel cell plate, comprising making a mold for processing the plate, on which a fluid flow space having a certain area and depth is formed on both sides, and a grid of fluid protruding into the fluid flow space is formed; making a plate using this mold; processing the plate to provide an inlet for fluid to flow into the meshed fluid flow space; and processing the plate to provide an outlet passage for fluid to flow out of the fluid flow space.
8. Bipolar fuel cell plate, containing a plate having a certain thickness and area; a channel area having lattice protrusions next to multiple lattice grooves formed along a certain area of both sides of the plate; an inlet port formed on a side of the plate connected to the lattice slots for introducing a fluid; and an outlet channel formed on a side of the plate connected to the grid slots to discharge fluid in the grid slots.
9. The bipolar plate of claim 8, wherein the grating protrusion is formed into a rectangular cone shape.
10. The bipolar plate of claim 8, wherein the lattice protrusions are formed at regular intervals.
11. The bipolar plate according to claim 8, wherein the inlet port and the outlet port are respectively formed on the side of the plate in an open shape with a certain width and depth.
12. The bipolar plate according to claim 8, the plate being made of a stainless steel material.
13. A method for manufacturing a bipolar fuel cell plate, including manufacturing a plate having a certain thickness and area; performing machining to form the lattice slots next to the lattice protrusions formed on both sides of the plate; and processing the plate with the inlet channel and the outlet channel connected to the lattice grooves.
14. The method according to claim 13, in which the stage of machining includes sub-steps: notches on both sides of the plate to form lattice protrusions; and grinding both notched sides of the plate.
15. A bipolar fuel cell plate, comprising: a plate having a certain thickness and area, in which, on both sides in the middle, numerous channels are formed by pressing, consisting of numerous ascents and descents so that they have a certain width and length; and a sealing member respectively attached to the contour of both sides of the plate so as to form internal channels, together with the plate channels, an inlet channel and an outlet channel through which fluid flows in and out of the channels.
16. Bipolar plate according to claim 15, in which the internal channels include an inlet buffer channel for distributing fluid through the channels of the plate; an outlet buffer channel for allowing fluid passing through the channels of the plate to flow into the outlet channel; and a connecting channel for connecting the inlet buffer channel and the outlet buffer channel.
17. A method of manufacturing a bipolar fuel cell plate, including cutting the plate so that it has a certain size; press processing both sides of the cut plate so as to form multiple channels through which the fluid flows; and combining the sealing element with the contour of the press-worked plate.
18. The method according to claim 17, wherein during the pressing step, the rises formed by the channels are processed so that they have the same height.
The invention relates to the field of electrical engineering and can be used in fuel cells







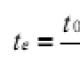


For beginners: breeding a broiler at home Boiled water for broilers
Only lovers will survive
Features of advertising aimed at children
retouching old photos in photoshop retouching old photos
What is an NPO: decoding, definition of goals, types of activities Does a non-profit organization have the right